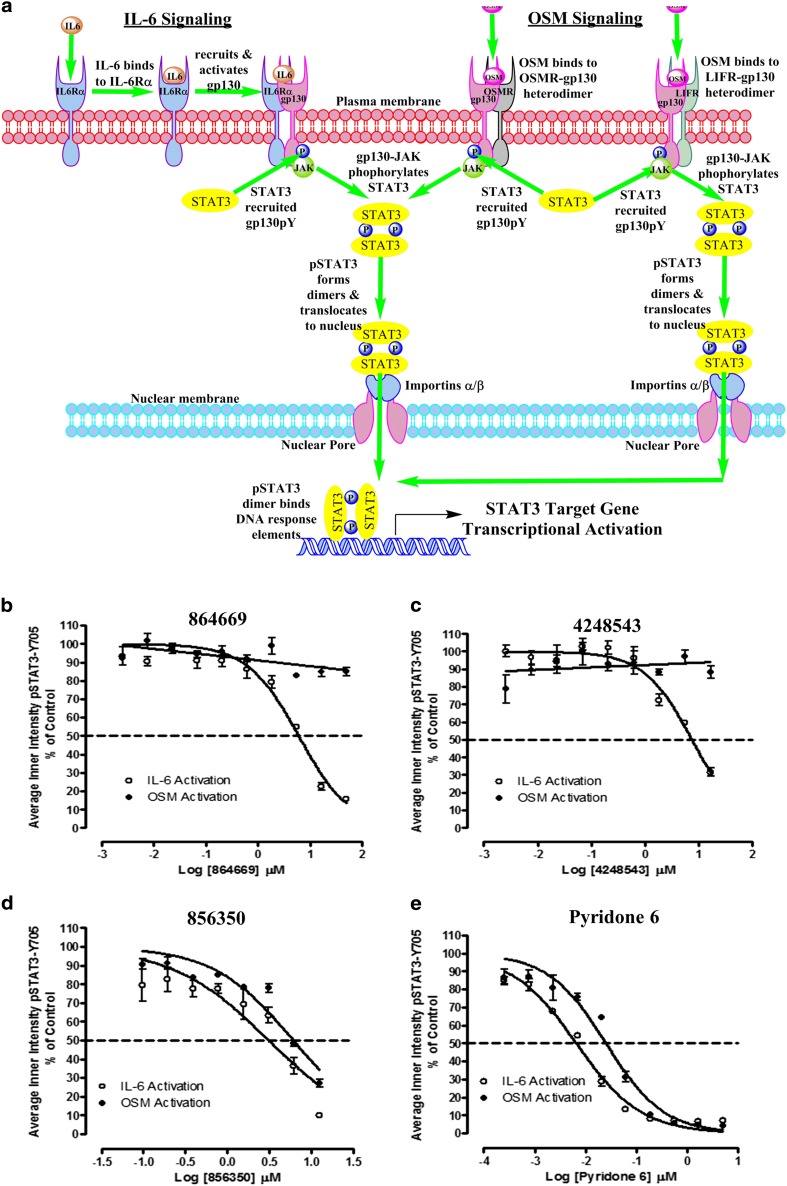
Fig. 4.
a STAT3 activation pathways for interleukin-6 and oncostatin M. In classical IL-6 signal transduction, IL-6 binds to IL-6 receptor alpha subunits (IL-6Rα, gp80) on cells that express this receptor, and this binary complex then recruits a gp130 signaling subunit to form an IL-6/IL-6Rα/gp130 heterotrimer and then two of these interact to form a hexamer-activated IL-6/IL-6Rα/gp130 signaling complex [13, 14, 26]. Homo-dimerization of the IL-6/IL-6Rα/gp130 heterotrimers triggers phosphorylation of the gp130-associated Janus kinase, and subsequent phosphorylation of tyrosine residues on the intracellular cytoplasmic tail of gp130 generates docking sites for the SH2 domains of STAT3 [13, 14, 26, 50]. JAK-mediated phosphorylation of the tyrosine-705 residue of STAT3 recruited to the activated IL-6/IL-6Rα/gp130/JAK complex enables reciprocal interactions between SH2 domains of pSTAT3 monomers to form dimers which then translocate into the nucleus [13, 14, 17, 26, 50]. In the nucleus, pSTAT3 dimers bind to specific DNA response elements in the promoters that activate the transcription of target genes that favor the development, progression, and maintenance of HNSCC and many other tumors [7, 10, 15, 17, 25]. In contrast, oncostatin M (OSM) can bind to two distinct heterodimer receptor complexes without first binding to an alpha receptor subunit and then recruiting gp130 [13, 14]. OSM initiates signal transduction by binding to heterodimer complexes formed between a gp130 subunit and either the OSM (OSMR) or leukemia inhibitory factor (LIFR) signaling receptors [13, 14]. Activation of either the IL-6/IL-6Rα/gp130/JAK, OSM/OSMR/gp130/JAK, or OSM/LIFR/gp130/JAK receptor complex triggers identical downstream signal transduction events with respect to STAT3 recruitment, tyrosine-705 phosphorylation of STAT3, pSTAT3 dimerization, and trafficking to the nucleus and transcriptional regulation of STAT3 target genes. b–e Inhibition of IL-6-induced versus OSM-induced pSTAT3Tyr705 activation by b 864669 and c 4248543 but not d 856350 and e Pyridone 6. Three hundred eighty-four-well plates were seeded with Cal33 HNSCC cells and serum starved for 24 h. The cells were exposed to the indicated concentrations of the selected compounds for 3 h and then treated with either 50 ng/ml of IL-6 or 50 ng/ml OSM for 15, fixed and stained with Hoechst and a mouse monoclonal anti-pSTAT3Tyr705 antibody. Images were acquired on the ImageXpress Ultra confocal HCS platform. To calculate the percent inhibition of IL-6-induced pSTAT3 activation, the mean average inner pSTAT3Tyr705 intensity values of the 0.2% DMSO minimum plate control wells (n = 32) and the 50 ng/ml IL-6 or OSM maximum plate control wells (n = 32) were used to normalize the mean average inner intensity pSTAT3Tyr705 values of the compound-treated wells and to represent 100 and 0% inhibition of IL-6-induced pSTAT3 activation, respectively. The mean ± SD (n = 3) percent inhibition of IL-6-induced pSTAT3 activation (open circle) and the mean ± SD (n = 3) percent inhibition of OSM-induced pSTAT3 activation (filled circle) at the indicated compound concentrations are presented. Representative experimental data from one of numerous independent experiments are shown