Key Figure, Figure 1. Homeostatic Responses to Tissue Stress in the Context of Pancreatic Dysfunction and Malignancy.
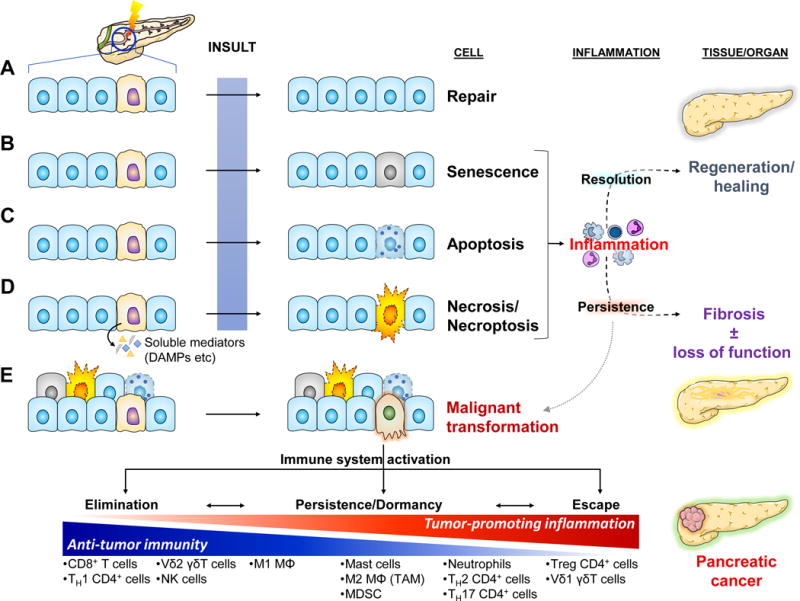
Cellular stress can be invoked by a great variety of internal (e.g. wear and tear/aging, reactive oxygen species, hypoxia, etc) and external causes (e.g. toxins, radiation, infectious agents, trauma, etc). Some can be mutagenic, potentially leading to cancer. Stressed/damaged cells activate defensive systems that attempt to repair the injury. If successful, the cell returns to its normal state and usually minimal or no inflammatory response is induced (a).
(b–d) If the injury is extensive and irreparable, the cell may either go into a senescence – a “safe mode” characterized by cell cycle arrest and slowing of cellular functions [101] – or may activate programmed cell death by apoptosis or necroptosis. If the injury is sudden and overwhelming, it may induce cell rupture and resultant necrosis. Both senescence and the various modes of cell death are characterized by altered expression of cell surface molecules as well as release of soluble mediators that activate the immune system [101, 102]. The resultant inflammatory response attempts to clear off the debris and support tissue regeneration. If the normal homeostatic mechanisms malfunction, the inflammation may persist leading to a futile cycle of further tissue injury, fibrosis, and potentially carcinogenesis.
(e) Malignant transformation usually arises de novo secondary to serial mutagenic insults to normal cells. The immune system usually recognizes single transformed cells and clears them off (elimination). Occasionally, some transformed cells may go unrecognized by the immune system and persist. As they accumulate additional mutations, they may initiate an inflammatory response that can clear them off. At the same time though, the inflammatory response places a selection pressure on the transformed cells which may enable the emergence of “resistant” clones and eventual escape from immunosurveillance mechanisms [103]. One of the ways the immune system escapes is by maintaining an immune infiltrate permissive to tumor growth through release of pro-tumorigenic mediators and simultaneous exclusion of cytotoxic cells (see Fig. 4). Finally, the sustained intratumoral inflammatory response may induce collateral damage to surrounding non-transformed epithelial cells. Activation of the responses to cell injury described above leads to release of additional pro-inflammatory mediators and further perpetuation of tumor-associated inflammation.
