Figure 5. Dysregulated Fibroblast Function in PDAC Leads to Desmoplasia where Cancer Cells Thrive.
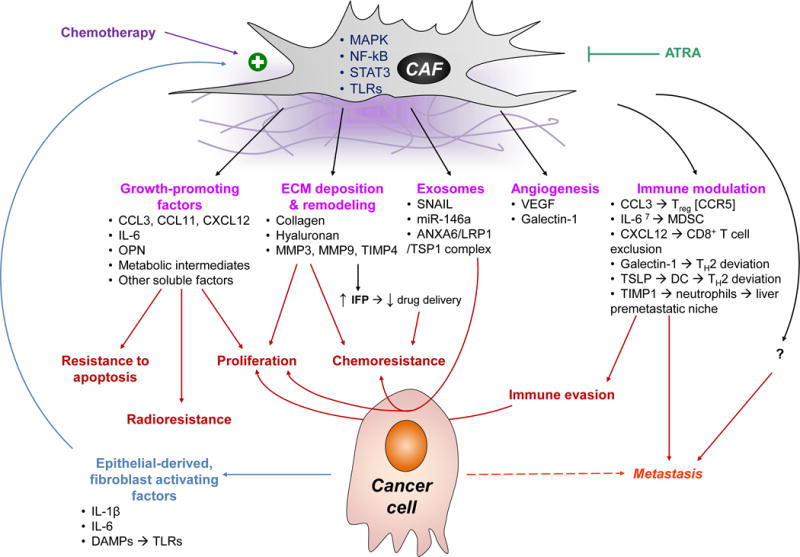
CAFs have a bidirectional communication with the epithelial compartment of the tumor. Epithelial-derived factors such as cytokines and chemokines, as well as DAMPs that ligate PRRs promote their activation via the MAPK, NF-κB, and STAT3 pathways [17, 18, 71, 104]. CAFs in turn provide, pro-proliferative and anti-apoptotic signals [18, 71, 105, 106]; promote angiogenesis [71, 104, 107]; modulate the ECM; and contribute to chemo- and radio-resistance [105, 106]. Importantly, CAFs also engage in crosstalk with immune cells and promote tumor tolerance: they recruit immunosuppressive cells such as Treg and MDSC [18, 104]; they skew helper T cells to TH2 deviation [25, 107, 108]; they sequester cytotoxic CD8+ T cells away from cancer cells [38, 51]; and recruit neutrophils to distant organs such as the liver, generating pre-metastatic niches [98, 99]. A newly appreciated mode of intercellular communication is exosomes, which are upregulated on CAFs under stressful conditions, such as hypoxia and exposure to chemotherapy [106, 109]. All the aforementioned mechanisms make the TME more hospitable to cancer cells, and contribute to tumor growth, immune escape, and metastasis. Agents such as all-trans retinoic acid (ATRA) may have a role in blocking their activation and disrupting their pro-tumorigenic function [110].
IFP, interstitial fluid pressure; MMP, matrix metalloprotease; OPN, osteoprotegerin; TSLP; thymic stromal lymphopoietin; VEGF, vascular endothelial growth factor.
