Table 1.
CBB-3CR involving tert-butylisocyanide.
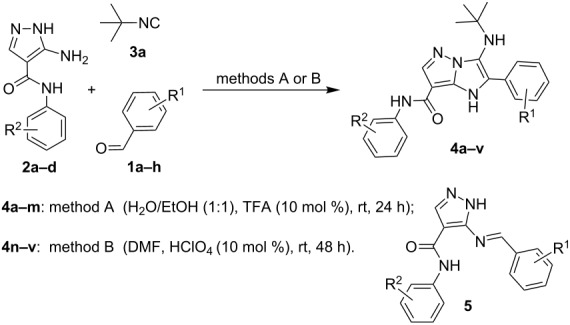 | |||||||
| Entry | Starting materials | Method | Product | Yield, % | |||
| Aldehydes | R1 | Aminopyrazoles | R2 | ||||
| 1 | 1a | H | 2a | 4-F | A | 4a | 54 |
| 2 | 1b | 2-CH3O | 2a | 4-F | A | 4b | 75 |
| 3 | 1c | 3-CH3O | 2a | 4-F | A | 4c | 77 |
| 4 | 1d | 4-CH3O | 2a | 4-F | A | 4d | 75 |
| 5 | 1e | 4-Cl | 2a | 4-F | A | 4e | 72 |
| 6 | 1b | 2-CH3O | 2b | 3-F | A | 4f | 83 |
| 7 | 1d | 4-CH3O | 2b | 3-F | A | 4g | 64 |
| 8 | 1e | 4-Cl | 2b | 3-F | A | 4h | 89 |
| 9 | 1b | 2-CH3O | 2c | 2-CH2CH3 | A | 4i | 82 |
| 10 | 1d | 4-CH3O | 2c | 2-CH2CH3 | A | 4j | 64 |
| 11 | 1e | 4-Cl | 2c | 2-CH2CH3 | A | 4k | 66 |
| 12 | 1b | 2-CH3O | 2d | 4-CH2CH3 | A | 4l | 85 |
| 13 | 1e | 4-Cl | 2d | 4-CH2CH3 | A | 4m | 53 |
| 14 | 1f | 4-CO2CH3 | 2a | 4-F | B | 4n | 85 |
| 15 | 1g | 4-NO2 | 2a | 4-F | B | 4o | 87 |
| 16 | 1h | 4-CN | 2a | 4-F | B | 4p | 90 |
| 17 | 1f | 4-CO2CH3 | 2b | 3-F | B | 4q | 88 |
| 18 | 1g | 4-NO2 | 2b | 3-F | B | 4r | 87 |
| 19 | 1h | 4-CN | 2b | 3-F | B | 4s | 82 |
| 20 | 1f | 4-CO2CH3 | 2c | 2-CH2CH3 | B | 4t | 61 |
| 21 | 1g | 4-NO2 | 2c | 2-CH2CH3 | B | 4u | 79 |
| 22 | 1h | 4-CN | 2c | 2-CH2CH3 | B | 4v | 83 |
