Table 4.
Reaction scope of the visible light-mediated coupling of arenediazonium tetrafluoroborates 1 with CS2 (2).
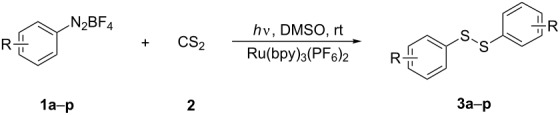 | |||
| Substrate 1a | Product 3, yieldb | Substrate 1a | Product 3, yieldb |
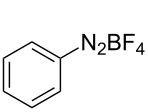 1a |
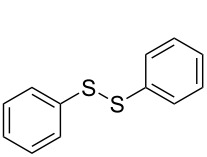 3a, 80%, 50%c |
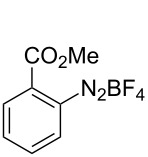 1i |
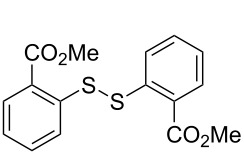 3i, 94%, 82%c |
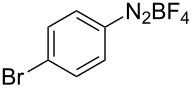 1b |
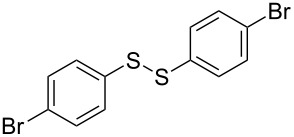 3b, 81%, 78%c |
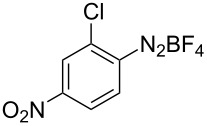 1j |
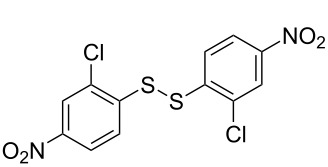 3j, 99%, 85%c |
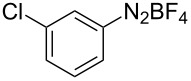 1c |
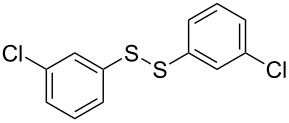 3c, 85%, 72%c |
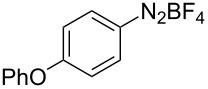 1k |
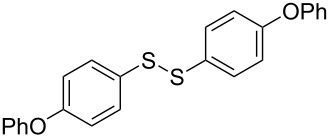 3k, 70% |
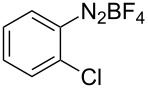 1d |
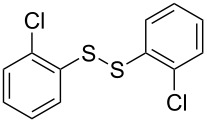 3d, 94% |
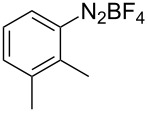 1l |
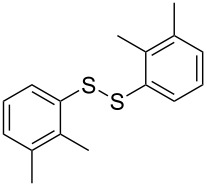 3l, 76% |
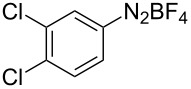 1e |
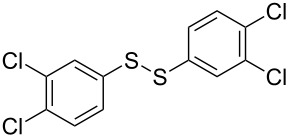 3e, 90% |
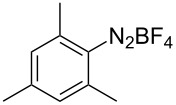 1m |
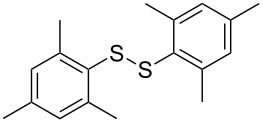 3m, 56% |
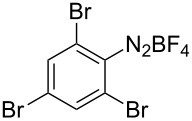 1f |
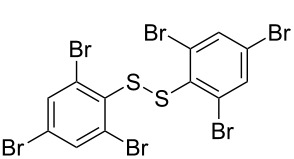 3f, 88% |
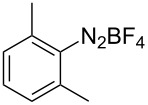 1n |
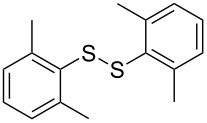 3n, 42% |
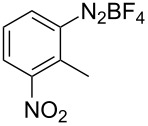 1g |
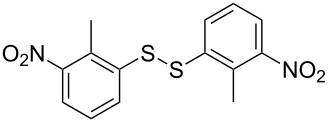 3g, 88% |
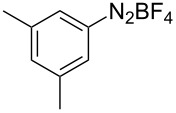 1o |
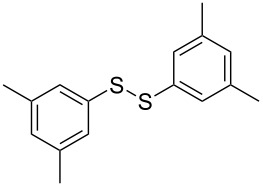 3o, 56% |
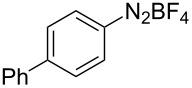 1h |
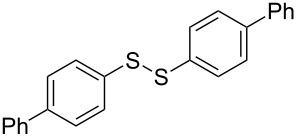 3h, 76% |
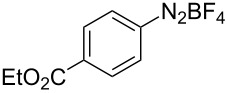 1p |
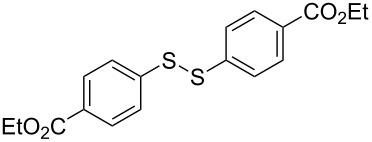 3p, 90%c, 92%c,d |
aReaction conditions: 1 (0.1 mmol), CS2 (0.2 mmol), Ru(bpy)3(PF6)2 (0.001 mmol), blue light (20 W), DMSO (2 mL), rt, 6 h; bisolated yields after chromatography on silica gel; cthe reactions were carried out with the diazonium salts 1 at a 5 mmol scale; dacetone was used as the solvent.
