Table 3.
Application of ring-closing metathesis reactions in the synthesis of macrocycles.
| Substrate | Methoda (mol %; time; yield) | RCM product |
| 3a | A (5; 2 h; 63%) B (5; 2 h; 84%) |
 4a |
| 3b | A (5; 2 h; 85%) B (5; 2 h; 94%) |
 4b |
| 3c | A (5; 2 h; 88%) B (5; 3 h; 94%) |
 4c |
| 3d | A (5; 2 h; 70%) B (5; 2 h; 90%) |
 4d |
| 3e | A (5+5; 3 h; 88%) B (5+3; 3 h; 40%) |
 4e |
| 3f | A (5+3; 3 h; 83%) B (5; 2 h; 39%) |
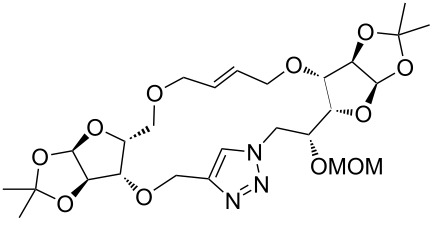 4f |
| 3g | A (5; 2 h; 77%) B (5+3; 3 h; 92%) |
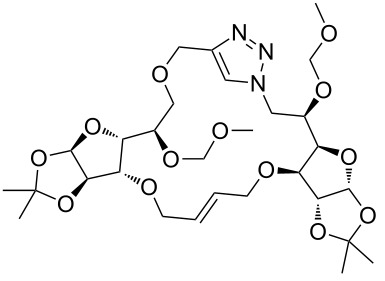 4g |
| 3h | A (5+3; 3 h; 82%) B (5; 2 h; 92%) |
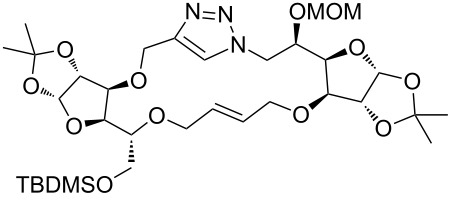 4h |
| 3i | A (5; 2 h; 95%) B (5; 2 h; 19%) |
 4i |
| 3j | A (5; 2 h; 84%) B (5; 2 h; 56%) |
 4j |
| 3k | A (5; 2 h; 81%) B (5; 2 h; 96%) |
 4k |
| 3l | A (5; 2 h; 53%) B (5; 2 h; 61%) |
 4l |
| 3m | A (5; 2 h; 40%) B (5; 2 h; 55%) |
 4m |
aMethods: A: Grubbs second-generation catalyst, CH2Cl2, 50 °C; B: Grubbs second-generation catalyst, ethyl acetate, 75 °C.
