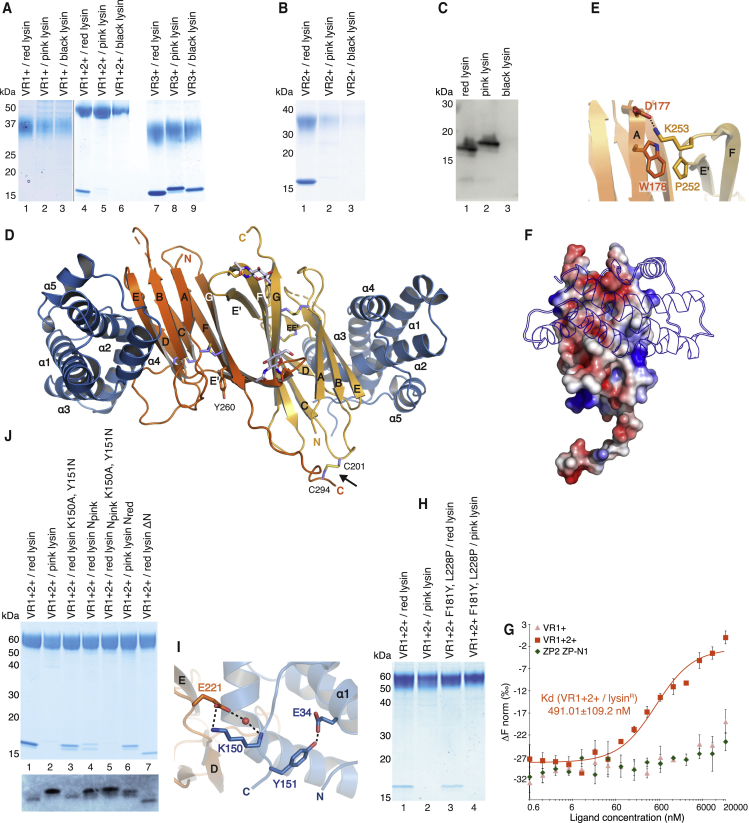
Figure 6.
VR2/Lysin Recognition Contributes to the Species-Specificity of Gamete Interaction
(A–B) His-pull-down analysis of lysins from different species of abalone co-expressed with (A) VR1+, VR1+2+, VR3+ or (B) VR2+ constructs of red abalone VERL. Like VR1+2+ (A, lane 4), VR2+ binds efficiently only to red abalone lysin (B, lane 1); however, different from VR1+2+, VR2+ is not well secreted by mammalian cells when it does not form a complex (B, lanes 2 and 3).
(C) Polyclonal anti-lysin immunoblot analysis of the secretion levels of individually expressed lysins.
(D) Cartoon representation of the crystal structure of the VR2+/lysin complex, with the two moieties of the VR2+ homodimer and lysin colored orange/yellow and blue, respectively. The intermolecular disulfide stabilizing the VR2+ homodimer is indicated by an arrow.
(E) Details of non-covalent interactions mediating VR2+ homodimerization.
(F) As observed in the case of VR3 (Figure 5B), the surface of VR2+ is electrostatically complementary to lysin.
(G) MST analysis of red lysinR interaction with red VR1+2+ and VR1+, as well as negative control ZP2 ZP-N1. Results are shown as mean ± SD.
(H) Amino acid substitutions converting the interface of red VR2 to that of pink do not change the species-specificity of the interaction between VR1+2+ and lysin.
(I) Close-up of the VR2+/lysin complex structure, showing how the C-terminal region of red lysin interacts with the de loop of VR2+.
(J) His-pull-down analysis of VR1+2+ co-transfected with N- and C-terminal variants of red and pink lysin. Bottom: An immunoblot with anti-lysin, showing the secretion levels of the same lysin constructs expressed individually.
See also Figures S5 and S7, and Tables S4 and S6.