Figure 7.
The Repeated Structure of VERL Filaments Suggests a Mechanism for Egg Coat Recognition and Penetration by Sperm
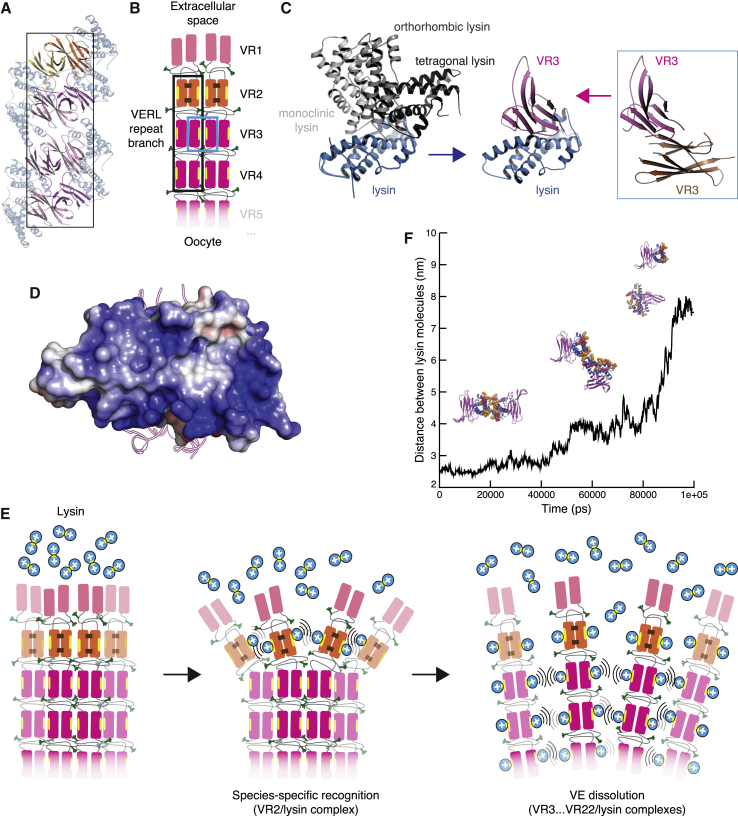
(A) Stacked VR2+ homodimers adopt a filament-like arrangement in the VR2+/lysin complex crystal. The boxed area suggests the repeat organization outlined in (B).
(B) Possible architecture of VERL repeat branches. Filled rectangles represent individual repeats, colored as in the previous figures and connected by flexible interdomain linkers (thin gray lines). Brown staples represent VR2+ intermolecular disulfide bonds. Yellow ellipses and green tripods indicate hydrophobic lysin-binding sites and O-glycans, respectively. Stacking of homodimeric VERL repeats within each branch is based on the packing shown in (A) (black rectangle); lateral interaction between repeats of adjacent branches is supported by the VR3/VR3 contacts depicted in (C) (blue rectangle).
(C) Surfaces mediating VR3/lysin interaction (center) make homomeric contacts in crystals of isolated lysin (left) and VR3 (right).
(D) As exemplified by the VR3/lysin complex structure, VERL repeat-bound lysin exposes a highly basic surface to the solvent.
(E) Proposed mechanism of VE recognition and non-enzymatic dissolution by lysin. Positively charged lysin molecules are represented by blue circles, with the hydrophobic VERL repeat-binding site shown as a yellow ellipse.
(F) 100 ns molecular dynamics simulation of two facing lysin molecules bound to VR3 repeats representing adjacent branches of VERL. Strong electrostatic repulsion between the exposed basic surfaces of the lysin molecules causes the complexes to be pushed apart over time. Basic surface Lys and Arg residues are in sphere representation and colored red and orange, respectively.
See also Figures S3 and S7, Tables S1, S3, and S4, and Movie S1.