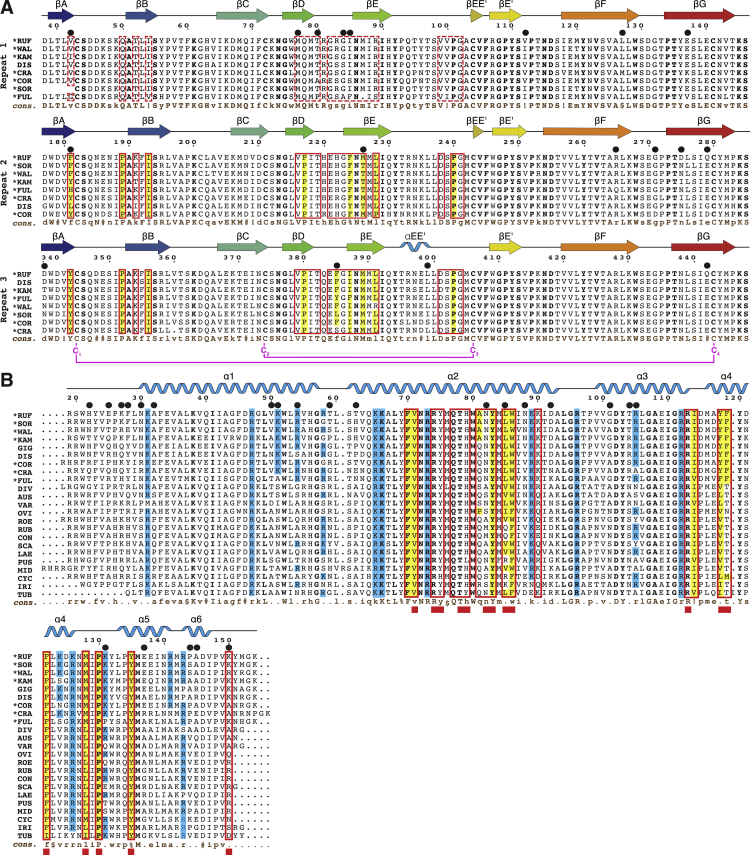
Figure S5.
Sequence Comparison of VERL Repeats and Lysin from Different Haliotis Species, Related to Figures 3 and 6
(A) Multiple sequence alignment, showing the intra- and interspecies similarity of VR1, VR2 and VR3. Sequences are listed in order of decreasing similarity to red abalone VERL. Secondary structure, based on the crystal structures of red abalone VR1+, VR2+ (from chain B of the VR2+/lysin complex) and VR3 (from the trigonal VR3/lysin complex), is depicted on top; consensus sequences are shown at the bottom (cons., brown). Conserved ZP-N Cys are marked and their connectivity is indicated (magenta). Interface residues of the VR3/lysin and VR2+/lysin complexes are indicated by red boxes; corresponding positions in VR1 are outlined by dashed red boxes. Hydrophobic interface residues are highlighted in yellow. Positively selected residues (Galindo et al., 2003, Kresge et al., 2001) are marked by black circles above the alignment. Abalone species abbreviations are: RUF, H. rufescens (red); SOR, H. sorenseni (white); WAL, H. walallensis (flat); KAM, H. kamtschatkana (pinto); GIG, H. gigantea (giant); DIS, H. discus hannai (Japanese); COR, H. corrugata (pink); CRA, H. cracherodii (black); FUL, H. fulgens (green); DIV, H. diversicolor (variously colored); AUS, H. australis (Australian); VAR, H. varia (variable); OVI, H. ovina (sheep’s ear); ROE, H. roei (Roe’s); RUB, H. rubra (blacklip); CON, H. conicopora; SCA, H. scalaris (staircase); LAE, H. laevigata (greenlip); PUS, H. pustulata; MID, H. midae (perlemoen); CYC, H. cyclobates (whirling); IRI, H. iris (paua); TUB, H. tuberculata (green ormer). Californian species (Lee and Vacquier, 1995) are marked by an asterisk next to their name abbreviation. Consensus symbols: uppercase, high consensus (> 90%); lowercase, low consensus (> 50%); !, I or V; $, L or M; %, F or Y; #, N or D or Q or E.
(B) Multiple sequence alignment of lysin. Conventions are as in (A), with interface residues of the VR3/lysin and VR2+/lysin complexes indicated by open red boxes in the alignment and closed red boxes below the consensus line, respectively. The secondary structure of red abalone lysin (from the trigonal VR3/lysin complex) is displayed, and solvent-exposed basic residues that do not interact with VR3 or VR2+ in the complex structures are highlighted in blue.