Abstract
Differentiation between the specific immunoglobulin G (IgG) response to Toxoplasma gondii by a mother and her newborn child is helpful in the diagnosis of congenital infection with T. gondii in newborns without T. gondii-specific IgM and/or IgA antibodies at birth. Previous methods include immunoblotting and complexing T. gondii antigen with the sera from the mother and child and comparing the bands after electrophoresis. We developed a two-dimensional immunoblotting (2DIB) method with T. gondii RH strain tachyzoite antigen and validated the method with sera from 11 children identified through the neonatal screening program for congenital toxoplasmosis in Denmark. The children were identified by using Toxoplasma-specific IgM antibodies at the screening test, but the presence of T. gondii-specific IgM and/or IgA antibodies could not be confirmed at the subsequent serum sample tested. The children were monitored for at least 12 months, and in seven of eight patients monitored for 12 months the results of the 2DIB-predicted congenital infection were confirmed by the presence of persistent Toxoplasma-specific IgG antibodies. 2DIB is a sensitive technique that allows early differentiation between passively transferred maternal T. gondii-specific IgG antibodies and antibodies synthesized by the newborn child.
Primary infection with Toxoplasma gondii during pregnancy may result in congenital infection of the fetus. The majority of congenitally infected newborns do not show clinical signs at birth but may later develop retinochoroiditis and reduced vision that can be prevented by early treatment (7). Diagnosis of congenital infection with T. gondii is difficult at birth if T. gondii-specific immunoglobulin M (IgM) and/or IgA antibodies are not present because present diagnostics methods can distinguish between maternal and fetal IgG only with difficulty.
Congenital toxoplasmosis is treated for up to 12 months continuously with sulfadiazine and pyrimethamine, which may cause side effects, and it is therefore important that the diagnosis of congenital toxoplasmosis is firmly established before treatment is started.
The detection of T. gondii-specific nucleic acid by PCR is possible in amniotic fluid if a primary T. gondii infection is diagnosed during pregnancy, but in countries without prenatal screening programs or with neonatal screening programs PCR-based diagnosis is not appropriate.
Neonatal screening for congenital toxoplasmosis is performed in New England, Denmark, and parts of Brazil by analyzing the blood samples obtained on filter paper (Guthrie cards) on day 5 postpartum (5, 6, 9). Detection of T. gondii-specific IgM antibodies eluted from the filter paper is followed by a request of a blood sample from both the mother and infant for confirmatory testing. Approximately 15 to 20% of these sera have been found to be negative for Toxoplasma-specific IgM (3, 8).
Previous studies have shown that transferred maternal and neonate-synthesized T. gondii-specific IgG antibodies can be differentiated by immunoblotting or immunocomplexing (1, 4, 12, 14). Immunoblotting identifies newborns with congenital toxoplasmosis with a sensitivity of ca. 70% (13, 16); this sensitivity increases to 85% within the first 3 months of life (4, 13, 16). These results still leave 15 to 30% of congenitally infected newborns without a confirmed diagnosis.
To improve the diagnosis of congenital toxoplasmosis, a two-dimensional immunoblotting (2DIB) assay was developed that can distinguish between maternal and neonate Toxoplasma-specific IgGs with a better sensitivity than previous assays.
MATERIALS AND METHODS
Patients.
Eleven newborn and their mothers were included based on the detection of T. gondii-specific IgM or IgA antibodies in an eluate from the filter paper samples obtained at day 5 after birth (15). The presence of Toxoplasma-specific IgM-antibodies was followed by a request for a serum sample for confirmatory tests from both the mother and the child, taken between 2 to 5 weeks postpartum.
Confirmatory tests included analysis of Toxoplasma-specific IgG and IgM antibodies by the VIDAS system (bioMérieux) and the modified ISAGA assay (bioMérieux), IgA enzyme-linked immunosorbent assay (Bio-Rad), and the Sabin-Feldman's dye test (in house).
2DIB antigen preparation.
T. gondii RH strain tachyzoites were prepared from T. gondii cultured in vitro in Vero cells by using Dulbecco modified Eagle medium-HEPES supplemented medium (Gibco/Life Technologies; catalog no. 041-911278M) supplemented with 5% fetal calf serum, 2 mM l-glutamine (Seromed), 0.1% Dextran T70 (Pharmacia Biotech), and 1 μg of gentamicin (Gibco)/ml.
The antigen was prepared from lysed, T. gondii tachyzoites (2). In brief, Vero cells were infected 3 days prior to harvest. When the cells started to rupture, releasing free tachyzoites, the culture was washed, and the supernatants containing the free tachyzoites were filtered through a 3-μm-pore-size polycarbonate filter, washed twice in phosphate-buffered saline (1,500 × g for 20 min at 4°C), pelleted by centrifugation at 16,000 × g for 3 min at 4°C, and stored in batches of 108 tachyzoites at −80°C.
Separation of antigen by two-dimensional electrophoresis, followed by immunoblotting.
Antigen preparation was carried out as previously described (2) with minor modifications. In brief, frozen tachyzoite pellets were redissolved in 40 μl of buffer (8 M urea, 4% CHAPS {3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate}, and 40 mM Tris), followed by three rounds of freeze-thawing at −80°C and 5 min of sonication in a water bath sonicator at 20°C; 350 μl of buffer containing 8 M urea, 2% CHAPS, and 25 mM dithiothreitol and 2% IPG buffer were added sufficient for rehydration into three strips of 7-cm Immobiline Drystrips (pH 4 to 7).
The first and second dimensions were separated by using a Multiphor II electrophoresis system (2), and ExcelGel XL SDS 12-14 gels were used for the second dimension. Drystrips were placed side by side flanked by two pieces of filter papers (2 by 4 mm) soaked in 10 μl of prestained sodium dodecyl sulfate-polyacrylamide gel electrophoresis standards (Bio-Rad). In some experiments, the gels were Coomassie blue or silver stained. Except when mentioned, all reagents and equipments for two dimensions were from Amersham Pharmacia Biotech.
The separated proteins were transferred to nitrocellulose membranes for 2 h and 30 min at 450 mA by using the Hoefer TE 77 semidry transfer unit.
Detection of IgG antibody profile.
Each membrane was cut to cover the size marker, and the first 6 cm of the area was exposed to the Drystrip. The membranes were blocked for 16 h with 3% bovine serum albumin in phosphate-buffered saline at 4°C, washed three times with wash buffer (0.1 M Tris base, 0.17 M NaCl, and 0.05% [vol/vol] Tween 20), and incubated 4 h at room temperature, followed by 16 h at 4°C with sera from the mother or child diluted 1:50 in wash buffer. The assay could be performed with a minimum of 166 μl of sera from each patient.
During the development of the assays, we found that especially the length of incubation with sera was crucial to obtain the strongest signal-to-noise ratio on the membrane. Also, the combination of room temperature and 4°C incubation improved the signal.
The membranes were washed three times, incubated with alkaline phosphatase-conjugated rabbit anti-human IgG (Dako catalog no. D0336) 1 h at room temperature and developed with nitroblue tetrazolium and BCIP (5-bromo-4-chloro-3-indolylphosphate) for 7 min.
Antibody profiles were analyzed by manual detection and automated spot detection by using the Z3 two-dimensional gel image analysis system (Compugen).
Conventional Western blotting for IgG profiling is, at our laboratory, routinely sent to J. Franck, Laboratoire de Parasitologie, Groupe Hospitalier de la Timone, Marseille, France.
RESULTS
Toxoplasma-specific IgG profiles from 11 newborns suspected of congenital toxoplasmosis were investigated with the 2DIB technique. Mother and child Toxoplasma-specific IgG profiles were compared to demonstrate antigens recognized only by sera from the child and not from the mother as a sign of neonate-synthesized T. gondii-specific IgG antibodies in the child compatible with congenital infection with T. gondii. All samples from both mother and child had Toxoplasma-specific IgG antibodies as determined by enzyme immunoassay (Vidas; bioMérieux).
Ten newborns were included because Toxoplasma-specific IgM antibodies were found in the eluate from the filter paper, and one child was included because of known Toxoplasma infection in the mother during pregnancy.
Sera from 8 of the 11 children showed positive 2DIB results, with spots not found in the 2DIB results from their mothers (Table 1). Two children were lost to follow-up, one of which was included only because of known seroconversion of the mother during pregnancy, and one was considered positive, with clinical symptoms and IgM and IgA present at birth. One child had persistent IgG at 1 year of age but negative 2DIB results at 1 month of age.
TABLE 1.
Characterization of cases with suspected and confirmed diagnosis of congenital toxoplasmosisa
| Case | IgM | IgA | IgG persistencec | 2D IBd | Clinical symptom | Western blot |
|---|---|---|---|---|---|---|
| 1 | + | + | NT | + | Retinochoroiditis | NT |
| 2 | − | +b | − | − | None | − |
| 3 | − | − | + | + | None | + |
| 4 | − | + | + | + | Unknowne | − |
| 5 | + | + | + | + | Retinochoroiditis | NT |
| 6 | − | − | + | + | None | + |
| 7f | − | − | NT | − | Unknown | NT |
| 8 | + | + | + | + | Unknown | NT |
| 9 | + | + | + | + | None | NT |
| 10 | − | − | + | − | None | NT |
| 11 | − | − | + | + | Unknown | NT |
+, Antibody positive; −, antibody negative; NT, not tested (except as noted [see footnote d]).
IgA positive at day 5 of life and IgA negative at day 40 of life.
At 12 months of age.
+, Unique spots present when incubated with child sera; −, spot pattern identical.
Child not examined or data not available.
Included in the study because of known seroconversion of the mother during pregnancy.
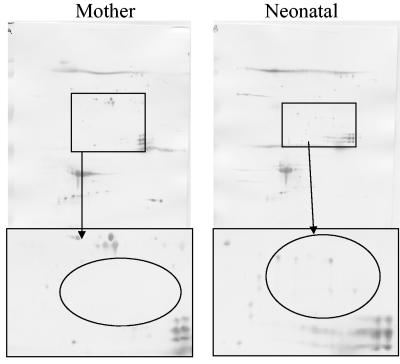
Of the 10 newborns with IgM positivity in the filter-paper assay, 6 were negative for Toxoplasma-specific IgM antibodies in the subsequent blood samples obtained a month later. Five of these six newborns were found to be congenitally infected as determined by the presence of T. gondii-specific IgG antibodies at 1 year of age. In one of these cases (case 4), a standard high-resolution Western blot (11) did not reveal a unique IgG profile for the child (Table 1). In contrast, the 2DIB technique showed several IgG-reactive antigens in the 2DIB results from the child that were not seen in the 2DIB with serum from the mother (Fig. 1).
FIG. 1.
2DIB with paired mother and infant sera from a child congenitally infected with T. gondii. Antigens recognized by the child's sera but not by the mother's sera show that Toxoplasma-specific IgG antibodies synthesized in the child have different specificities than the maternal Toxoplasma-specific IgG antibodies.
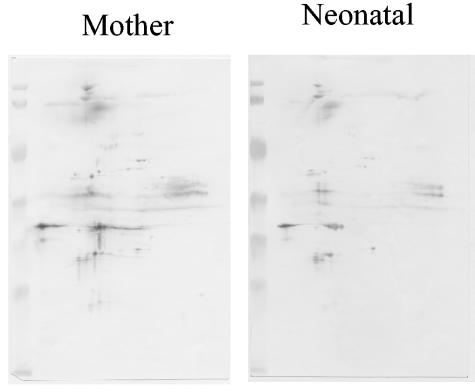
Figure 2 shows an example of a paired mother-child IgG profile, in which the child was not infected with T. gondii. The figure illustrates that the profile of a noninfected child is a copy of the mother's profile, with the reduced intensity of the former profile reflecting a lower level of Toxoplasma-specific IgG in the child.
FIG. 2.
2DIB analysis with serum from a T. gondii-noninfected child. The Toxoplasma-specific IgG profiles of the child and the mother are identical.
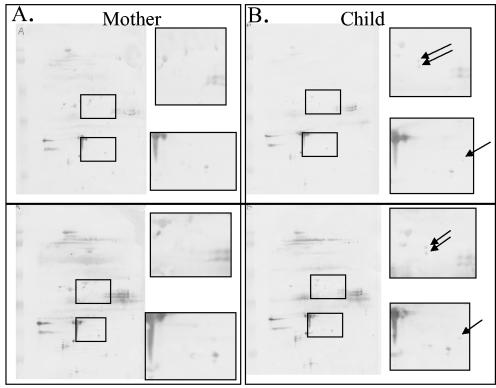
The 2DIB technique is technically difficult, and reproducibility was studied at different time points with different antigen batches. Coomassie blue-stained two-dimensional gels were compared, and the reproducibility was found to be very high (data not shown). Furthermore, the 2DIB analysis was performed twice on 5 of the 11 samples for which sufficient sera were available.
Figure 3 shows a representative example of two mother-child pairs analyzed twice by 2DIB. The color intensity, especially for regions in which antigen smears were found, may be variable between two separate runs, but individual spots outside these regions are easily identified at the same coordinates.
FIG. 3.
Representative 2DIB results from the same mother-child pair illustrate the reproducibility of this test. The color intensity, especially for regions where antigen smears are present, may vary between two separate runs, but individual spots outside these regions are clearly identified at the same coordinates.
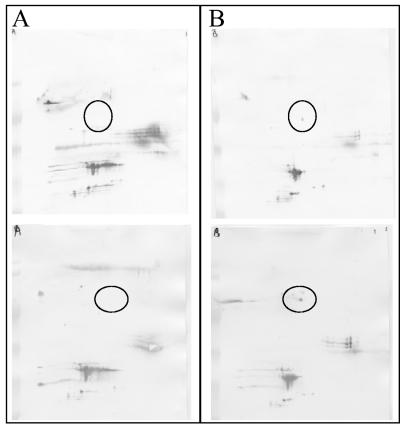
A difference between the mother's and the child's IgG profiles of a single spot was seen in one child (Fig. 4); however, the child had Toxoplasma-specific IgG antibodies at 12 months of age, demonstrating that, even with such a small difference between the mother and the child, 2DIB showed that the child was infected.
FIG. 4.
2DIB from a mother-infant pair with only a single spot difference between the 2DIB pattern of the mother (A and C) and the infant (B and D). The infant was confirmed congenitally infected as demonstrated by the presence of Toxoplasma-specific IgG antibodies at 12 months of age.
DISCUSSION
The diagnosis of congenital toxoplasmosis is often difficult and sometimes cannot be confirmed before 12 months of age. If the child has been treated continuously with sulfadiazine and pyrimethamine, the synthesis of Toxoplasma-specific IgG antibodies can be suppressed, and the serological confirmation of the infection cannot be made with certainty before or during the second year of life (17). This situation is found when T. gondii infection is suspected but Toxoplasma-specific IgM and/or IgA antibodies cannot be demonstrated in the child and when parasitological investigations, such as PCR analysis for Toxoplasma-specific nucleic acid, are negative or not appropriate.
Demonstration of Toxoplasma-specific IgG antibodies with different specificities in sera from the mother and child show that the child synthesizes its own IgG antibodies, confirming that the child is infected with T. gondii.
Differentiation of the specificities of IgG antibodies in the mother and child can also be done by comparing Toxoplasma-specific IgG profiles by conventional, one-dimensional immunoblotting or complexing of T. gondii antigen with sera before electrophoresis of the antigen-antibody complex is performed (1, 10, 11, 14). The two methods were compared in a double-blind study and found to be equally sensitive (11). However, both methods failed to correctly diagnose approximately one-third of children with congenital toxoplasmosis analyzed within 3 months of birth.
The 2DIB methodology greatly increased the resolution of the antibody response by allowing identification of up to a thousand spots, whereas the most sensitive Western blots do not allow distinction of more than 50 bands and often considerably fewer.
The drawbacks of 2DIB are its technical complexity and the requirement of a relatively larger amount of sera. However, the method allows diagnosis within a few weeks to months after birth in patients who otherwise would have to wait for up to 1 year of age, or even longer in children receiving long-term treatment, before the infectious state was known with certainty.
In one case (case 10) the maternal and offspring Toxoplasma-specific IgG antibody levels were >500 IU/ml, and in this situation it was not possible to identify unique spots in the sera from the child. In this patient the sera from the mother recognized numerous T. gondii antigens, which may leave little space for child-specific spots.
For national reference laboratories that currently use one-dimensional immunoblot or immunocomplexing techniques, the 2DIB technique provides a new option for improving diagnosis of congenital toxoplasmosis in the newborn child within the first few months after birth.
Acknowledgments
We thank Lis Wassman for skillful technical assistance.
REFERENCES
- 1.Chumpitazi, B. F., A. Boussaid, H. Pelloux, C. Racinet, M. Bost, and A. Goullier-Fleuret. 1995. Diagnosis of congenital toxoplasmosis by immunoblotting and relationship with other methods. J. Clin. Microbiol. 33:1479-1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen, A. M., K. Rumpel, G. H. Coombs, and J. M. Wastling. 2002. Characterization of global protein expression by two-dimensional electrophoresis and mass spectrometry: proteomics of Toxoplasma gondii. Int. J. Parasitol. 32:39-51. [DOI] [PubMed] [Google Scholar]
- 3.Decoster, A., F. Darcy, A. Caron, D. Vinatier, D. Houze de L'Aulnoit, G. Vittu, G. Niel, F. Heyer, B. Lecolier, M. Delcroix, et al. 1992. Anti-P30 IgA antibodies as prenatal markers of congenital toxoplasma infection. Clin. Exp. Immunol. 87:310-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gross, U., C. G. Luder, V. Hendgen, C. Heeg, I. Sauer, A. Weidner, D. Krczal, and G. Enders. 2000. Comparative immunoglobulin G antibody profiles between mother and child (CGMC test) for early diagnosis of congenital toxoplasmosis. J. Clin. Microbiol. 38:3619-3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guerina, N. G., H. W. Hsu, H. C. Meissner, J. H. Maguire, R. Lynfield, B. Stechenberg, I. Abroms, M. S. Pasternack, R. Hoff, R. B. Eaton, et al. 1994. Neonatal serologic screening and early treatment for congenital Toxoplasma gondii infection. N. Engl. J. Med. 330:1858-1863. [DOI] [PubMed] [Google Scholar]
- 6.Lebech, M., O. Andersen, N. C. Christensen, H. E. Nielsen, B. Peitersen, C. Rechnitzer, S. O. Larsen, B. Norgaard-Pedersen, and E. Petersen. 1999. Neonatal screening for congenital infection with Toxoplasma gondii in Denmark. Lancet 353:1834-1837. [DOI] [PubMed] [Google Scholar]
- 7.McAuley, J., K. M. Boyer, D. Patel, M. Mets, C. Swisher, N. Roizen, C. Wolters, L. Stein, M. Stein, W. Schey, et al. 1994. Early and longitudinal evaluations of treated infants and children and untreated historical patients with congenital toxoplasmosis: the Chicago Collaborative Treatment Trial. Clin. Infect. Dis. 18:38-72. [DOI] [PubMed] [Google Scholar]
- 8.Naot, Y., G. Desmonts, and J. S. Remington. 1981. IgM enzyme-linked immunosorbent assay test for the diagnosis of congenital Toxoplasma infection. J. Pediatr. 98:32-36. [DOI] [PubMed] [Google Scholar]
- 9.Neto, E. C., R. Rubin, J. Schulte, and R. Giugliani. 2004. Newborn screening for congenital infectious diseases. Emerg. Infect. Dis. 10:1068-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pinon, J. M., C. Chemla, I. Villena, F. Foudrinier, D. Aubert, D. Puygauthier-Toubas, B. Leroux, D. Dupouy, C. Quereux, M. Talmud, T. Trenque, G. Potron, M. Pluot, G. Remy, and A. Bonhomme. 1996. Early neonatal diagnosis of congenital toxoplasmosis: value of comparative enzyme-linked immunofiltration assay immunological profiles and anti-Toxoplasma gondii immunoglobulin M (IgM) or IgA immunocapture and implications for postnatal therapeutic strategies. J. Clin. Microbiol. 34:579-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pinon, J. M., H. Dumon, C. Chemla, J. Franck, E. Petersen, M. Lebech, J. Zufferey, M. H. Bessieres, P. Marty, R. Holliman, J. Johnson, V. Luyasu, B. Lecolier, E. Guy, D. H. Joynson, A. Decoster, G. Enders, H. Pelloux, and E. Candolfi. 2001. Strategy for diagnosis of congenital toxoplasmosis: evaluation of methods comparing mothers and newborns and standard methods for postnatal detection of immunoglobulin G, M, and A antibodies. J. Clin. Microbiol. 39:2267-2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Remington, J. S., P. Thulliez, and J. G. Montoya. 2004. Recent developments for diagnosis of toxoplasmosis. J. Clin. Microbiol. 42:941-945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rilling, V., K. Dietz, D. Krczal, F. Knotek, and G. Enders. 2003. Evaluation of a commercial IgG/IgM Western blot assay for early postnatal diagnosis of congenital toxoplasmosis. Eur. J. Clin. Microbiol. Infect. Dis. 22:174-180. [DOI] [PubMed] [Google Scholar]
- 14.Robert-Gangneux, F., V. Commerce, C. Tourte-Schaefer, and J. Dupouy-Camet. 1999. Performance of a Western blot assay to compare mother and newborn anti-Toxoplasma antibodies for the early neonatal diagnosis of congenital toxoplasmosis. Eur. J. Clin. Microbiol. Infect. Dis. 18:648-654. [DOI] [PubMed] [Google Scholar]
- 15.Sorensen, T., J. Spenter, I. Jaliashvili, M. Christiansen, B. Norgaard-Pedersen, and E. Petersen. 2002. Automated time-resolved immunofluorometric assay for Toxoplasma gondii-specific IgM and IgA antibodies: study of more than 130,000 filter-paper blood-spot samples from newborns. Clin. Chem. 48:1981-1986. [PubMed] [Google Scholar]
- 16.Tissot Dupont, D., H. Fricker-Hidalgo, M. P. Brenier-Pinchart, C. Bost-Bru, P. Ambroise-Thomas, and H. Pelloux. 2003. Usefulness of Western blot in serological follow-up of newborns suspected of congenital toxoplasmosis. Eur. J. Clin. Microbiol. Infect. Dis. 22:122-125. [DOI] [PubMed] [Google Scholar]
- 17.Walloon, M., G. Cozon, R. Ecochard, P. Lewin, and F. Peyron. 2001. Serological rebound in congenital toxoplasmosis: long-term follow-up of 133 children. Eur. J. Pediatr. 160:534-540. [DOI] [PubMed] [Google Scholar]