Abstract
Aim:
To structurally modify our existing cholic acid (CA)-based telodendrimer (TD; PEG5K-CA8) for effective micellar nanoencapsulation and delivery of the US FDA-approved members of taxane family.
Materials & methods:
Generation of hybrid TDs was achieved by replacing four of the eight CAs with biocompatible organic moieties using solution-phase peptide synthesis. Drug loading was done using the standard evaporation method.
Results:
Hybrid TDs can generate micelles with narrow size distributions, low critical micelle concentration values (1–6 μM), better hematocompatibility and lack of in vitro cytotoxicity.
Conclusion:
Along with PEG5K-CA8, CA-based hybrid nanoplatform is the first of its kind that can stably encapsulate all three FDA-approved taxanes with nearly 100% efficiency up to 20% (w/w) loading.
Keywords: : cabazitaxel, cholic acid, docetaxel, drug delivery, micelle, nanoparticle, paclitaxel, taxane, telodendrimer
Taxanes are a benchmark class of small-molecule anticancer agents that work by interfering with normal microtubule breakdown during cell division [1–3]. Currently, three members of taxane family, namely paclitaxel (PTX), docetaxel (DTX) and cabazitaxel (CTX) have been approved by the US FDA for clinical use, with the first two being widely prescribed as front-line treatment options for many forms of cancers such as breast, ovarian and lung cancer [1,4–7]. CTX is the latest member of taxane family that has been approved for the treatment of hormone refractory prostate cancer [8]. Despite their widespread popularity, all three taxanes show very low solubility in water, thereby making development of effective formulations for medicinal use challenging. They are either formulated in a mixture of Cremophor EL/absolute ethanol or in Polysorbate 80, both of which are associated with serious side effects (hypersensitivity reactions, peripheral neurotoxicity, etc.) [9,10]. Patients receiving these drugs require premedication with steroids and benadryl. Application of nanomedicine in cancer field has led to the development and FDA approval of PTX-loaded human serum albumin nanoaggregates (Abraxane®) [11]. Although more drug can be given (240 mg/m2 PTX for Abraxane vs 175 mg/m2 PTX for paclitaxel), these nanoparticles are relatively ‘large’ (130 nm in diameter), and improvement in clinical efficacy is only marginal. A wide variety of newer PTX and DTX nanoformulations have been explored [12–14] such as NK105 [15] and Genexol-PM [16], which are under clinical development, and more recently CTX nanoformulations have been attempted [17,18]. Reports exist on encapsulation of PTX [19], DTX [20] and some third-generation taxoids (excluding CTX) [21] in poly(2-oxazoline)-based micelles. To the best of our knowledge, there is not a single polymer or nanoplatform that can stably encapsulate all the three FDA-approved members of taxane family with significant loading capacity and efficiency. We have previously reported polyethylene glycol (PEG) and cholic acid (CA)-based micellar system PEG5K-CA8 telodendrimer (TD) [22] that can load PTX with very high capacity and efficiency. Here, we report the design, synthesis and characterization of a small set of structurally related amphiphilic polymers capable of nanoformulating all three members of the taxane family, with high efficiency, but lower hemolytic potential.
Materials & methods
For synthesis and other experimental details, please see Supplementary Information file.
Results
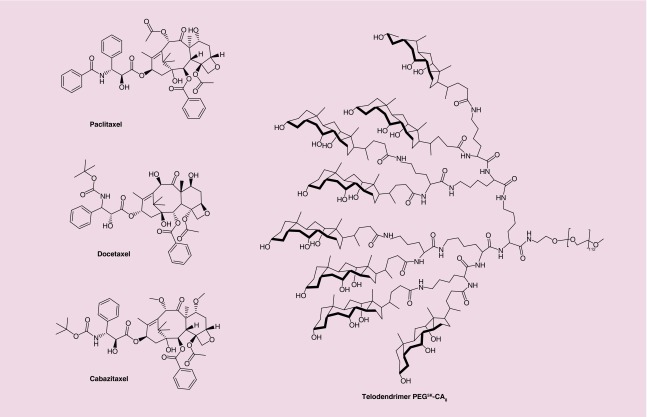
With the development of several nanocarriers based on liposomes, micelles, polymers, hydrogels etc., many hydrophobic cancer drugs can now be stably encapsulated and delivered site specifically to cancer site via active or passive targeting [14,23–27]. We have recently reported a robust micellar nanoparticle platform that can stably encapsulate a range of hydrophobic agents in the hydrophobic core [22,28–34]. This nanoplatform is assembled from amphiphilic TDs comprised of natural surfactant CA and dendritic lysines linked to a linear PEG, represented by the formula PEGnK-CAy (where n = molecular weight in kilodaltons [K], y = number of CA units). PEG5K-CA8 (Figure 1), one of our most studied TD can stably encapsulate a range of cancer drugs including PTX, a taxane [22], and a number of other chemotherapeutic agents. TD-encapsulated PTX with almost 100% loading efficiency when the initial amount of PTX was less than 25 wt% of PEG5K-CA8 (20 mg/ml), with superior stability (longer than 6 months) and a size of 20–60 nm [22]. Though PEG5K-CA8 showed superior loading capability with PTX, it failed to do so with DTX and CTX (see Tables 3 & 5). Given that all three taxanes are structurally quite similar (Figure 1), this result is somewhat surprising. It underscores the importance of matching the right nanocarrier with the intended drug payload.
Figure 1. . Chemical structures of FDA-approved taxanes and PEG5K-CA8 TD.
Table 3. . Docetaxel loading (1.5 mg/15 mg polymer, n = 1).
| Polymer | Particle size (volume%, width) (nm) | Drug/polymer (w/w) (%) | Loading efficiency (%) |
|---|---|---|---|
| PEG5K-CA8 |
13.3 (100, 8.01) |
10 |
69 |
| 1 |
10.5 (100, 7.91) |
10 |
74 |
| 2 |
12.9 (100, 7.88) |
10 |
100 |
| 3 |
19.6 (95, 16.67)/295.4 (5, 312) |
10 |
0 |
| 4 |
9.35 (100, 7.78) |
10 |
Precipitated |
| 5 |
11.8 (100, 10.3) |
10 |
100 |
| 6 |
10.8 (100, 8.3) |
10 |
77 |
| 7 |
13.6 (95.1, 12.53)/122.8 (4.9, 206.5) |
10 |
90 |
| 8 |
13 (100, 10.33) |
10 |
2 |
| 9 |
12.4 (93.9, 9.65)/338 (6.1, 226.5) |
10 |
61 |
| 10 |
12.3 (100, 7.67) |
10 |
100 |
| 11 |
11 (95.9, 9.42)/1087 (4.1, 2586) |
10 |
21 |
| 12 |
16.1 (100, 9.07) |
10 |
1 |
| 14 |
19.7 (100, 8.88) |
10 |
85 |
| 18 | 6.5 (19.8, 2)/11.3 (24.2, 5.4)/33.6 (50.5, 27.8)/173.6 (5.5, 109.9) | 10 | 18 |
PEG: Polyethylene glycol; CA: Cholic acid.
Table 5. . Cabazitaxel loading (2.0 mg/15 mg polymer, n = 1).
| Polymer | Particle size (volume%, width) (nm) | Drug/polymer (w/w) (%) | Loading efficiency (%) |
|---|---|---|---|
| PEG5K-CA8 |
15.8 (96.2, 33)/348 (3.8, 1983) |
13.35 |
79 |
| 1 |
23.9 (100, 28.9) |
13.35 |
57 |
| 2 |
15.9 (95.3, 7.13)/222.9 (4.7, 304) |
13.35 |
100 |
| 3 |
16.9 (15.4, 4.2)/49.8 (84.6, 74.4) |
13.35 |
38 |
| 4 |
20.73 (100, 12.15) |
13.35 |
100 |
| 5 |
0.99 (31, 0.22)/15.38 (69, 11.87) |
13.35 |
89 |
| 6 |
11.87 (100, 10.6) |
13.35 |
41 |
| 7 |
18.0 (100, 14.4) |
13.35 |
82 |
| 8 |
25.27 (100, 69.9) |
13.35 |
80 |
| 9 |
23.3 (100, 24.0) |
13.35 |
66 |
| 10 |
16.2 (100, 10.0) |
13.35 |
74 |
| 11 |
27.9 (95.3, 56.1)/1399 (4.7, 1193) |
13.35 |
31 |
| 12 |
23.98 (100, 27.6) |
13.35 |
70 |
| 14 |
0.96 (100, 0.21) |
13.35 |
55 |
| 18 | 84.4 (94.6, 70)/396 (5.4, 229.4) | 13.35 | 69 |
PEG: Polyethylene glycol; CA: Cholic acid.
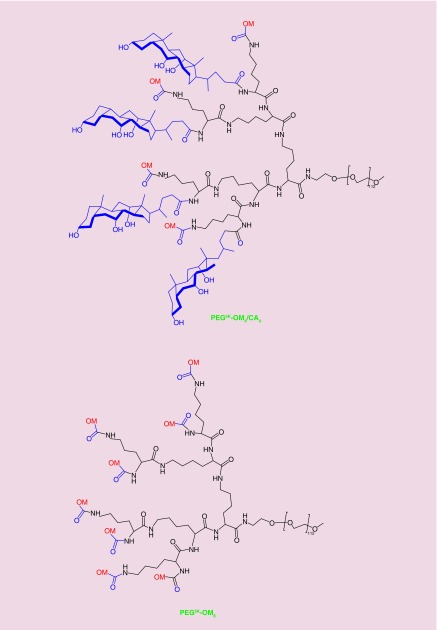
Versatility of our nanoplatform lies in its architecture that allows for easy structural modulation to meet the desired goal [35,36] such as substituting some CAs with organic moieties (OMs). We believe such modifications will enable us to generate novel TDs that are tailored toward specific drug or groups of drugs. Replacing four of the eight CAs in PEG5K-CA8 with OMs generated TDs (hybrid TDs), represented as PEG5K-OM4/CA4 (Figure 2). We also developed structural analogs of PEG5K-CA8, where all the eight CAs were replaced with OMs (polymers designated as PEG5K-OM8) to examine the contribution of CA to drug loading. All the polymers were prepared according to our well-established published procedures [22,36].
Figure 2. . Representative structures of the two types of amphiphilic polymers used in this study.
Characterization
Molecular weights of all the prepared polymers were measured with MALDI-TOF mass spectrometry. The molecular weights of the polymers (Table 1) matched closely with the theoretical values (Tables 1 & 2). The molecular weight distribution between the polymers and the corresponding starting PEG were almost identical (see representative examples in Supplementary Figure 3). Nuclear magnetic resonance (NMR) analysis was done to confirm grafting of cholane and OM on the PEG chain (Tables 1 & 2). Signals of PEG chain (3.6 ppm) and of CA methyl peaks at 0.66, 0.87 and 1.01 ppm, as previously used by our group [22,37], along with characteristic peaks of OM, were used for analysis (Supplementary Figure 4 for representative examples). Based on the average molecular weight (5000) of PEG chains, ratio of PEG protons to that of cholane and organic moieties was very close to the expected values, which indicated successful grafting. HPLC was used to assess the purity of the samples. Based on tert-butyloxycarbonyl (Boc)-protected starting material, newly prepared TDs showed chromatograms similar to Boc-PEG5K-NH2. Based on the heterogeneity of the starting material and samples very broad peak, it was difficult to judge degree of impurity of the final TD product (Supplementary Figure 5 for representative examples). The particle size of the micelles formed by self-assembly of the polymers in phosphate-buffered saline (PBS) is shown in Tables 1 & 2. Almost all the prepared hybrid polymers (Table 1) yielded particle sizes in the range of 7 to 32 nm with a narrow size distribution. Only TD 9 (PEG5K-[Retinoic acid]4/CA4) has a minor portion of aggregates (2000 nm). Moreover, all the prepared hybrid TDs have good water solubility (up to at least 15 mg/ml, data not shown) with critical micelle concentration (CMC) values ranging between 1 and 6 μM, as determined by using pyrene as a hydrophobic probe (Supplementary Figure 6 for representative example). Compared with hybrid TDs, nanoparticles assembled from PEG5K-OM8-based polymers showed a much broader size distributions (Table 2) and several of them generated heterogenous populations of nanoparticles with different sizes. Only PEG5K-(Biotin)8, PEG5K-(Glycocholic acid)8 and PEG5K-(Chenodeoxycholic acid)8 yielded particle size distributions comparable to those produced by the hybrid polymers. Several of them (entry 16, 17, 21 and 22) became poorly soluble with higher ionic strength (<0.25 mg/ml in PBS), and were not evaluated further for CMC values. CMC values are reported in Table 2, for the PEG5K-OM8 polymers with solubility ≥2 mg/ml. CMC values were found to be in the range of 2–22 μM. Based on the solubility (at least 15 mg/ml, data not shown), low CMC value and size distribution, only the following, among the PEG5K-OM8 group, were selected for further studies: PEG5K-(Biotin)8, PEG5K-(Glycocholic acid)8, PEG5K-(Chenodeoxycholic acid)8 and PEG5K-(Nicotinic acid)8. We selected 14 polymers (four PEG5K-OM8 and ten hybrid TDs) to determine particle size variation as a function of polymer concentration (0.5 and 5 mg/ml). Except for polymers 8 and 11 that generated significant size variations at the two different concentrations, the remaining polymers showed nearly similar size distribution (Supplementary Table 1).
Table 1. . Characterization of micelles formed by hybrid telodendrimers (PEG5K-OM4/CA4).
| Entry | OM | Polymer | Molecular.weight exp. (theo.) (Da) | NMR cholane:PEG:OM or cholane+OM:PEG obs (theo.) | CMC (μM) | Particle size (volume%, width) (nm) |
|---|---|---|---|---|---|---|
| 1 |
Biotin (vitamin H)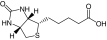 |
PEG5K-(Biotin)4/CA4 |
7984 (8416) |
1†:15.17 (1:12.44) |
3 |
11.2 (100, 7.13) |
| 2 |
trans-cinnamic acid (used as flavoring agent)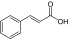 |
PEG5K-(Cinnamic acid)4/CA4 |
7758 (8033) |
1:15.27:0.42 (1:12.44:0.44‡) |
1 |
20.1 (100, 7.8) |
| 3 |
Lauric acid (saturated fatty acid)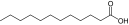 |
PEG5K-(Lauric acid)4/CA4 |
7919 (8241) |
1:11.28 (1§:9.33) |
3 |
20.8 (100, 32.7) |
| 4 |
Linoleic acid (polyunsaturated fatty acid)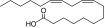 |
PEG5K-(Linoleic acid)4/CA4 |
8146 (8561) |
1:10.23 (1¶:9.33) |
1 |
31.2 (91.3, 21.4)/129.5 (8.7, 93.5) |
| 5 |
Lipoic acid (cofactor) |
PEG5K-(Lipoic acid)4/CA4 |
7819 (8265) |
1:13.65:0.19 (1:12.44:0.22#) |
2 |
11.8 (100, 10.3) |
| 6 |
Nicotinic acid (vitamin B3)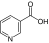 |
PEG5K-(Nicotinic acid)4/CA4 |
7788 (7931) |
1††:16.75 (1:12.44) |
4 |
10.6 (100, 6.37) |
| 7 |
Octanoic acid (saturated fatty acid)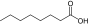 |
PEG5K-(Octanoic acid)4/CA4 |
7548 (8016) |
1:11.46 (1‡‡:9.33) |
2 |
10.7 (100, 11.6) |
| 8 |
Oleic acid (mono unsaturated fatty acid) |
PEG5K-(Oleic acid)4/CA4 |
8472 (8569) |
1:11.86 (1§§:9.33) |
3 |
15.9 (100, 22.7) |
| 9 |
Retinoic acid (vitamin A metabolite)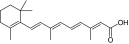 |
PEG5K-(Retinoic acid)4/CA4 |
7961 (8641) |
1:9.42 (1¶¶:7.47) |
6 |
7.9 (95.7, 7.69)/2048 (4.3, 720) |
| 10 | Sorbic acid (food preservative)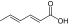
|
PEG5K-(Sorbic acid)4/CA4 | 7620 (7891) | 1:11.09 (1##:12.44) | 4 | 6.9 (89, 4.7)/32.1 (11, 15.9) |
†Biotin peaks either overlap with backbone or not strongly differentiable from background.
‡4H of cinnamic between 7.3 and 7.5 ppm.
§Lauric methyl along with cholane methyl.
¶Linoleic methyl along with cholane methyl.
#2H of lipoic between 3.12 and 3.18 ppm.
††1H proton peaks from nicotinic present but not strongly differentiable from background. Only cholane peak used.
‡‡Octanoic methyl along with cholane methyl.
§§Oleic methyl peak along with cholane methyl.
¶¶Two methyl present on quaternary carbon in retinoic cyclic ring along with cholane.
##Vinyl proton of sorbic present but not strongly differentiable from background. Only cholane peak used.
CA: Cholic acid; CMC: Critical micelle concentration; exp.: Experimental; NMR: Nuclear magnetic resonance; obs.: Observed; OM: Organic moiety; PEG: Polyethylene glycol; theo.: Theoretical.
Table 2. . Characterization of micelles formed by polymers (PEG5K-OM8).
| Entry | Polymer | Molecular weight exp. (theo.) (Da) | NMR† OM:PEG obs (theo.) | CMC (μM) | Particle size (volume%, width) (nm) |
|---|---|---|---|---|---|
| 11 |
PEG5K-(Biotin)8 |
7769 (7760) |
NC‡ |
2 |
12.5 (100, 10.8) |
| 12 |
PEG5K-(Chenodeoxycholic acid)8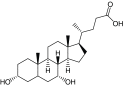 |
8600 (8946) |
1:5.99 (1:6.22) |
2 |
32.5 (100, 25.1) |
| 13 |
PEG5K-(Cinnamic acid)8 |
6994 (6991) |
1:17.78 (1:14) |
22 |
17.2 (97.5, 28.9)/5810 (2.5, 973) |
| 14 |
PEG5K-(Glycocholic acid)8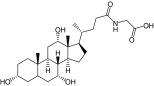 |
9472 (9531) |
1:7.01 (1:6.22) |
1 |
18.1 (100, 8.4) |
| 15 |
PEG5K-(Lauric acid)8 |
7445 (7409) |
1:5.62 (1§:3.5) |
13 |
10.3 (9.7, 2.3)/27.8 (90.3, 31) |
| 16 |
PEG5K-(Linoleic acid)8 |
N.O. (8050) |
1:15.79 (1:18.67) |
ND |
19.1 (97.4, 28.9)/5780 (2.6, 3170)‡‡ |
| 17 |
PEG5K-(Lipoic acid)8 |
N.O. (7457) |
N.O. |
ND |
Wide range of distribution from 21 to 5700 nm‡‡ |
| 18 |
PEG5K-(Nicotinic acid)8 |
6867 (6791) |
NC¶ |
3 |
1 (32.3, 0.3)/75.3 (56.3, 81)/377 (11.4, 229) |
| 19 |
PEG5K-(Octanoic acid)8 |
6915 (6960) |
1:9.39 (1#:7) |
3 |
15.3 (12.3, 5.2)/44.7 (82.7, 49.1)/5970 (5, 676) |
| 20 |
PEG5K-(Oleic acid)8 |
7944 (8066) |
1:21.85 (1:18.67) |
3 |
40.9 (89.4, 43.5)/1249 (3.3, 527)/5740 (7.3, 1096) |
| 21 |
PEG5K-(Retinoic acid)8 |
7800 (8209) |
1:11.66 (1:9.33) |
ND |
28 (89.9, 32.7)/456 (5.7, 298.7)/1842 (4.4, 1644)‡‡ |
| 22 | PEG5K-(Sorbic acid)8 | 6396 (6703) | NC†† | ND | 25.3 (86.2, 33.4)/5810 (13.8, 958)‡‡ |
†Peaks used that are different from Table 1, are summarized below.
‡NC: Not calculated. Biotin peaks either overlap with backbone or not strongly differentiable from background.
§8, CH2: 1.07–01.46 ppm.
¶1H proton peaks from nicotinic present but not strongly differentiable from background.
#4, CH2 peak around 1.29 ppm.
††Vinyl proton of sorbic present but not strongly differentiable from background.
‡‡Measured at around 0.2 mg/ml.
CMC: Critical micelle concentration; exp.: Experimental; ND: Not determined; NMR: Nuclear magnetic resonance; obs.: Observed; OM: Organic moiety; PEG: Polyethylene glycol; theo.: Theoretical.
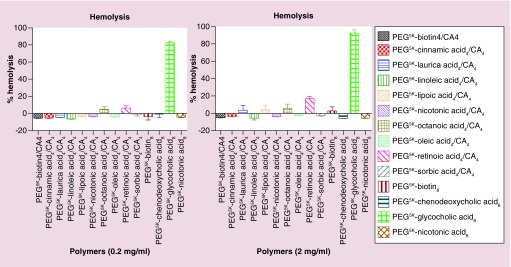
Hemolysis
Hemolysis induced by hybrid TDs and selected PEG5K-OM8 polymers were examined to gain insight into their hematocompatibility. Hemolysis was detected through spectrophotometric measurement of the hemoglobin present in the supernatant of red blood cells (RBCs) treated with the micellar nanoparticles. Previously, we have shown dose-dependent RBC lysis from standard TD PEG5K-CA8. Hemolysis increased from 9.0 to 16.3% when the concentration of PEG5K-CA8 was increased from 0.2 to 1.0 mg/ml, respectively [38]. For the current set of polymers, hemolytic studies were performed at concentrations of 0.2 and 2 mg/ml (expected blood levels during therapy). Figure 3 shows the observed hemolytic activity from the hybrid TDs and PEG5K-OM8 polymers. At 0.2 mg/ml, most of the polymers showed no hemolytic activity except PEG5K-(Glycocholic acid)8 polymer (81.9%). Hemolysis from PEG5K-(Octanoic acid)4/CA4 and PEG5K-(Retinoic acid)4/CA4 were significantly lower (4.0 and 5.5%, respectively). At higher polymer concentration (2 mg/ml), except for PEG5K-(Retinoic acid)4/CA4 that showed hemolysis in double digits (16.2%) and PEG5K-(Glycocholic acid)8 that showed even higher hemolysis (90.9%), most of the hybrid TDs and PEG5K-OM8 were either devoid of hemolytic activity or only induced minimal hemolysis (2.7, 3.4 and 4.7% for lauric-, lipoic- and octanoic-based hybrid TDs, respectively, and 1.9% for PEG5K-[Biotin]8). The high hemolytic activity of PEG5K-(Retinoic acid)4/CA4 and PEG5K-(Glycocholic acid)8 is of concern, but can probably be mitigated by introducing disulfide cross-links in the hydrophobic core as previously reported for our standard TD [38]. Hemolytic activity was also evaluated at 4 mg/ml for localized concentrations effect. Results were quite similar to 2 mg/ml, with marginally increased hemolysis from lauric- and octanoic-based hybrid TDs (Supplementary Figure 7).
Figure 3. . In vitro red blood cell (RBC) lysis from selected polymers. RBC incubation with Triton-100 (2%) and PBS were used as the positive and negative controls, respectively. Values reported are the mean ± SD for triplicate samples.
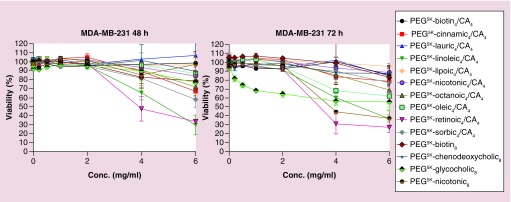
In vitro cytotoxicity
Empty nanoparticles assembled from hybrid TDs were evaluated for cytotoxicity against human breast cancer cells MDA-MB-231 using an MTS assay. As shown in Figure 4, the nanocarriers did not exhibit detectable cytotoxicity up to at least 2 mg/ml for incubation over 48 or 72 h, except for PEG5K-(Glycocholic acid)8, which showed some toxicity at the 72 h time point. When examined at even much higher levels (4 and 6 mg/ml), most of the polymers still lacked any significant toxicity. Notable exceptions are linoleic acid and retinoic hybrid, with less than 50% viability at 48 and 72 h, and PEG5K-Nicotinic8 at 72 h. Viability for PEG5K-(Glycocholic acid)8 was about 50% at 72 h. Lack of significant cytotoxicity from most of these micelles indicates their potential usage as drug carrier for nanoformulation.
Figure 4. . The cytotoxicity of empty hybrid TDs against MDA-MB-231 breast cancer cells. Values reported are the mean ± SD for triplicate samples.
Drug loading for DTX, CTX & PTX
Over the past several years, we have successfully loaded a number of hydrophobic drugs (PTX, doxoruicin, vincristine, daunorubicin, etc.) in our standard nanomicelles assembled from PEG5K-CA8 and these nanoformulations were proven to be efficacious in several xenograft models [22,28,30,32,39,40]. However, we have also found that PEG5K-CA8 does not work for all hydrophobic drugs such as DTX (Table 3), CTX (Table 5) and SN-38 (data not shown). In order to show versatility and flexibility of our nanomicelles, we evaluated a small set of polymers for their ability to encapsulate DTX (Tables 3 & 4) and CTX (Tables 5 & 6).
Table 4. . Docetaxel loading (3 mg/15 mg polymer; mean ± SD, n = 3).
| Polymer | Particle size (volume%, width) (nm) |
3-mg DTX |
|
|---|---|---|---|
| Drug/polymer (w/w) (%) | Loading efficiency (%) | ||
| 2 |
27.7 (100, 16.7) |
20 |
93 ± 2.9 |
| 5 |
27.3 (100, 22.9) |
20 |
87 ± 3.2 |
| 10 | 140.5 (39.5, 73.9)/443 (60.5, 235.9) | 20 | 57 ± 1.4 |
DTX: Docetaxel; SD: Standard deviation.
Table 6. . Cabazitaxel loading (3 mg/15 mg polymer; mean ± SD, n = 3).
| Polymer | Particle size (volume%, width) (nm) |
3-mg CTX |
|
|---|---|---|---|
| Drug/polymer (w/w) (%) | Loading efficiency (%) | ||
| 2 |
22 (100, 11.3) |
20 |
93 ± 4.9 |
| 4 | 37.5 (100, 29.9) | 20 | 98 ± 3.4 |
CTX: Cabazitaxel; SD: Standard deviation.
As shown in Table 3, loading efficiency of the standard TD PEG5K-CA8 for DTX at 10% drug:polymer ratio (w/w) was found to be 69%. At the very same drug:polymer ratio, six different hybrid TDs were found to have higher drug-loading efficiencies than that of PEG5K-CA8 (polymers 1, 2, 5, 6, 7 and 10). Among the PEG5K-CA8 class, PEG5K-(Glycocholic acid)8 has better loading efficiency than that of the standard TD. Other than PEG5K-(Octanoic acid)4/CA4 (polymer 7), all other favorable candidates had particle sizes less than 20 nm with narrow size distribution. Moreover three of them, namely polymer 2 (PEG5K-(Cinnamic acid)4/CA4); polymer 5 (PEG5K-(Lipoic acid)4/CA4) and polymer 10 (PEG5K-(Sorbic acid)4/CA4) encapsulated DTX with 100% efficiency. These three polymers were selected for loading at a higher drug concentration (Table 4). At DTX loading of 3/15-mg polymer, both 2 and 5 still retained the high drug-loading efficiency (93 and 87%, respectively), but loading efficiency of 10 was found to drop to 57%. Based on the DTX screening, we identified two polymers, 2 and 5, that can stably encapsulate DTX with significant loading capacity (nearly 20% w/w). Nanoformulations were considered stable if they did not form a visible precipitate after 48-h storage at 4°C and if they maintained nearly the same particle size (monitored by dynamic light scattering [DLS], data not shown).
CTX, another member of taxane family and recently approved for the treatment of prostate cancer was screened next. Compared with DTX, CTX had a better drug loading (Table 5) in standard TD PEG5K-CA8 (79% at 2.0 mg of CTX compared with 69% at 1.5 mg of DTX per 15 mg of polymer). Despite better loading of CTX than DTX by PEG5K-CA8, broader size distribution of the final nanoparticles was observed in CTX formulation. Compared with PEG5K-CA8, four hybrid TDs, namely 2, 4, 5 and 7, showed better loading efficiency while PEG5K-(Oleic acid)4/CA4 (polymer 8) showed similar loading efficiency. On the other hand, none of the PEG5K-OM8 polymers showed better drug loading than PEG5K-CA8. PEG5K-(Cinnamic acid)4/CA4 (polymer 2) and PEG5K-(Linoleic acid)4/CA4 (polymer 4), both of which showed 100% loading efficiency, were tested further for higher drug loading. Both of these hybrid TDs were found to retain nearly 100% loading efficiency at 3 mg of drug and 15 mg of polymer (Table 6). Based on CTX screening, two polymers with significant loading capacity (nearly 20% w/w) were identified and loading was even better when compared with the results of DTX. For representative DLS data on drug-loaded samples (Tables 3 & 5), see Supplementary Figure 8.
PEG5K-(Cinnamic acid)4/CA4, PEG5K-(Linoleic acid)4/CA4 and PEG5K-(lipoic acid)4/CA4 were also evaluated for PTX encapsulation. While PEG5K-(Linoleic acid)4/CA4 showed 100% loading efficiency only at 5% w/w, PEG5K-(Cinnamic acid)4/CA4 and PEG5K-(lipoic acid)4/CA4 showed 100% efficiency up to 15% w/w and 20% w/w loading, respectively (Supplementary Table 2 for particle size distribution).
Discussion
As the amphiphilic TDs are prepared by step-wise peptide-synthesis method, we can easily introduce different linkers, amphiphilic building blocks or OM to the termini of the PEG-oligolysine (or other diaminocarboxylic acid) dendrimer [35]. Here, we focused our effort on developing TDs that can encapsulate all three taxanes: PTX, DTX and CTX. To minimize toxicity from these newer TDs, we selected only those OMs that are either produced endogenously, consumed by human as part of their diet, or are FDA-approved drugs or food additives. Chemical structures of simple aliphatic or aromatic OMs used to prepare different hybrid TDs are shown in Table 1. For our initial study, we focused on small set of saturated and unsaturated fatty acids (entry 3, 4, 7 and 8), cyclic vitamins (entry 1, 6 and 9, with 1 and 6, also being heterocyclic compounds), a flavoring agent and food preservative (entry 2 and 10, respectively) and lastly a cofactor (entry 5, which is also heterocyclic compound). We also included natural surfactants chenodeoxycholic acid and glycocholic acid as an alternative to CA (Table 2, entry 12 and 14) for the polymer class PEG5K-OM8. Compared with majority of PEG5K-OM8, PEG5K-(OM)4/CA4 TDs displayed narrower size distribution, lower CMC values and better aqueous solubility. Our previously reported standard TD PEG5K-CA8 also has CMC value in the same range (5.3 μM) [28], indicating that substitution of four CAs with other organic blocks has minor effects on the CMC values. Hematocompatibility is necessary for clinical translation of polymer-based drug carriers. Amphiphilic polymers have the potential to cause disruption of the plasma membrane, particularly of RBCs. When tested for their hemolytic potential, hybrid TD (except retinoic acid one), behaved much more like control, PBS, at 2 mg/ml concentration indicating their usefulness as drug carriers. Moreover, lack of any apparent cytotoxicity from majority of these TDs further reinforces their nanocarrier potential. When tested for their drug-loading potential, several TDs outweighed PEG5K-CA8 in terms of loading efficiency (Tables 3 & 5). Of the 15 TDs (PEG5K-(OM)4/CA4 and PEG5K-OM8) evaluated for DTX and CTX loading, two from each group were found to have significant loading capacity. Loading efficiency was nearly 100% when the initial amount of DTX or CTX used was less than 20 wt% of the polymer, a result matching to our prior observation with PTX loading by PEG5K-CA8. Nanoformulations were stable with narrow size distribution. These data indicate that CA, compared with other OMs, has a unique physicochemical property such that its presence at 50% level at the dendron is able to maintain the stability, monodisperse property and small size (<50 nm) of the nanocarrier, and to stably encapsulate hydrophobic drugs. It appears that the amphiphilic nature of the rigid, disc-shaped tetracyclic CA molecule, with three hydroxyl groups facing one side and hydrophobic ring surfaces facing the other, may allow the CAs to assemble as a stable barrier between the hydrophilic PEG corona and hydrophobic core of the nanocarrier. On special note, based on our result so far, PEG5K-(Cinnamic acid)4/CA4 seems to be the best TD of the lot as it stably encapsulated all members of taxane family with significant loading. Also, our data suggest hybrid system to be much more robust and efficient when compared with PEG5K-OM8, again signifying the importance of CA moiety in the scaffold. In summary, we have generated a small set of robust TDs that can be synthesized easily. We are currently testing these nanoformulations in various mouse models for their in vivo therapeutic efficacy and toxicity. We are also screening these polymers and other new hybrid TDs for their capacity to load other hydrophobic drugs that have poor loading capacity with our standard polymer. We believe that this systematic experimental approach will enable us to tailor specific TDs for nanoformulation of many current and future toxic drugs, thus enhancing their therapeutic index and efficacy.
Conclusion
The modular CA-based micellar nanocarrier platform allows systematic incorporation of additional organic moieties into the amphiphilic polymer, such that the resulting hybrid TDs can be tailored for a wide range of otherwise hard to nanoformulate hydrophobic drugs. Here, we have shown their utility in successful nanoformulation of approved taxane with significant loading capacity.
Executive summary.
Synthesis of PEG5K-OM4/CA4 & PEG5K-OM8
Solution-phase peptide synthesis was used to synthesize 22 new polymers.
Physicochemical properties
Molecular weights of prepared polymers match with theoretical values. NMR reveals successful grafting of cholane and organic moiety. Hybrid telodendrimers have narrow size distribution, similar CMC values and better aqueous solubility compared with majority of PEG5K-OM8.
Hemolysis & in vitro toxicity
Out of the 14 polymers tested for hemolytic potential, 12 of them showed minimal or no hemolysis at 2 mg/ml concentration.
When tested for potential cytotoxicity, almost all the polymers tested (up to 2 mg/ml) showed no toxicity up to 72 h.
Drug loading
PEG5K-(Cinnamic acid)4/CA4 and PEG5K-(Lipoic acid)4/CA4 showed nearly 100% loading for docetaxel (3 mg drug/15 mg polymer).
PEG5K-(Cinnamic acid)4/CA4 and PEG5K-(Linoleic acid)4/CA4 showed nearly 100% loading for cabazitaxel (3 mg drug/15 mg polymer).
PEG5K-(Cinnamic acid)4/CA4 also showed 100% loading for paclitaxel (2.25 mg drug/15 mg polymer).
Supplementary Material
Footnotes
Financial & competing interests disclosure
The authors thank NIH for the funding support: 3R01CA115483, 1U01CA198880-01 to KS Lam and 5R01CA199668 to Y Li. G Bharadwaj, Y Li and KS Lam are the inventors of a pending patent on hybrid TDs (US Patent Application no. 62/395,237). KS Lam is the founding scientist of LamnoTherapeutics, Inc., which plan to develop the nanotherapeutics described in the manuscript. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
All animals were kept under pathogen-free conditions according to AAALAC guidelines. All animal experiments were performed in compliance with institutional guidelines and according to protocol approved by the Animal Use and Care Administrative Advisory Committee at the University of California, Davis. The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations.
References
- 1.Rowinsky EK. The development and clinical utility of the taxane class of antimicrotubule chemotherapy agents. Annu. Rev. Med. 1997;48:353–374. doi: 10.1146/annurev.med.48.1.353. [DOI] [PubMed] [Google Scholar]
- 2.Yared JA, Tkaczuk KH. Update on taxane development: new analogs and new formulations. Drug Des. Devel. Ther. 2012;6:371–384. doi: 10.2147/DDDT.S28997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Y-F, Shi Q-W, Dong M, Kiyota H, Gu Y-C, Cong B. Natural taxanes: developments since 1828. Chem. Rev. 2011;111(12):7652–7709. doi: 10.1021/cr100147u. [DOI] [PubMed] [Google Scholar]
- 4.De Weger VA, Beijnen JH, Schellens JH. Cellular and clinical pharmacology of the taxanes docetaxel and paclitaxel--a review. Anti-Cancer Drugs. 2014;25(5):488–494. doi: 10.1097/CAD.0000000000000093. [DOI] [PubMed] [Google Scholar]
- 5.Bedard PL, Di Leo A, Piccart-Gebhart MJ. Taxanes: optimizing adjuvant chemotherapy for early-stage breast cancer. Nat. Rev. Clin. Oncol. 2010;7(1):22–36. doi: 10.1038/nrclinonc.2009.186. [DOI] [PubMed] [Google Scholar]
- 6.Chu Q, Vincent M, Logan D, Mackay JA, Evans WK. Taxanes as first-line therapy for advanced non-small cell lung cancer: a systematic review and practice guideline. Lung Cancer. 2005;50(3):355–374. doi: 10.1016/j.lungcan.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 7.Guastalla Iii JP, Dieras V. The taxanes: toxicity and quality of life considerations in advanced ovarian cancer. Br. J. Cancer. 2003;89(Suppl 3):S16–S22. doi: 10.1038/sj.bjc.6601496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bishr M, Saad F. Overview of the latest treatments for castration-resistant prostate cancer. Nat. Rev. Urol. 2013;10(9):522–528. doi: 10.1038/nrurol.2013.137. [DOI] [PubMed] [Google Scholar]
- 9.Reddy LH, Bazile D. Drug delivery design for intravenous route with integrated physicochemistry, pharmacokinetics and pharmacodynamics: illustration with the case of taxane therapeutics. Adv. Drug Del. Rev. 2014;71:34–57. doi: 10.1016/j.addr.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 10.Joerger M. Treatment regimens of classical and newer taxanes. Cancer Chemother. Pharmacol. 2016;77(2):221–233. doi: 10.1007/s00280-015-2893-6. [DOI] [PubMed] [Google Scholar]
- 11.Gradishar WJ. Albumin-bound paclitaxel: a next-generation taxane. Expert Opin. Pharmacother. 2006;7(8):1041–1053. doi: 10.1517/14656566.7.8.1041. [DOI] [PubMed] [Google Scholar]
- 12.Jäger E, Jäger A, Chytil P, et al. Combination chemotherapy using core-shell nanoparticles through the self-assembly of HPMA-based copolymers and degradable polyester. J. Control. Release. 2013;165(2):153–161. doi: 10.1016/j.jconrel.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 13.Bushman J, Vaughan A, Sheihet L, Zhang Z, Costache M, Kohn J. Functionalized nanospheres for targeted delivery of paclitaxel. J. Control. Release. 2013;171(3):315–321. doi: 10.1016/j.jconrel.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 14.Wang AZ, Langer R, Farokhzad OC. Nanoparticle delivery of cancer drugs. Annu. Rev. Med. 2012;63:185–198. doi: 10.1146/annurev-med-040210-162544. [DOI] [PubMed] [Google Scholar]
- 15.Kato K, Chin K, Yoshikawa T, et al. Phase II study of NK105, a paclitaxel-incorporating micellar nanoparticle, for previously treated advanced or recurrent gastric cancer. Invest. New Drugs. 2012;30(4):1621–1627. doi: 10.1007/s10637-011-9709-2. [DOI] [PubMed] [Google Scholar]
- 16.Lee KS, Chung HC, Im SA, et al. Multicenter Phase II trial of Genexol-PM, a cremophor-free, polymeric micelle formulation of paclitaxel, in patients with metastatic breast cancer. Breast Cancer Res. Treat. 2008;108(2):241–250. doi: 10.1007/s10549-007-9591-y. [DOI] [PubMed] [Google Scholar]
- 17.Bensaid F, Thillaye Du Boullay O, Amgoune A, et al. Y-shaped mPEG-PLA cabazitaxel conjugates: well-controlled synthesis by organocatalytic approach and self-assembly into interface drug-loaded core-corona nanoparticles. Biomacromolecules. 2013;14(4):1189–1198. doi: 10.1021/bm400161g. [DOI] [PubMed] [Google Scholar]
- 18.Zhou G, Jin X, Zhu P, et al. Human serum albumin nanoparticles as a novel delivery system for cabazitaxel. Anticancer Res. 2016;36(4):1649–1656. [PubMed] [Google Scholar]
- 19.Luxenhofer R, Schulz A, Roques C, et al. Doubly amphiphilic poly(2-oxazoline)s as high-capacity delivery systems for hydrophobic drugs. Biomaterials. 2010;31(18):4972–4979. doi: 10.1016/j.biomaterials.2010.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han Y, He Z, Schulz A, et al. Synergistic combinations of multiple chemotherapeutic agents in high capacity poly(2-oxazoline) micelles. Mol. Pharm. 2012;9(8):2302–2313. doi: 10.1021/mp300159u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He Z, Schulz A, Wan X, et al. Poly(2-oxazoline) based micelles with high capacity for 3rd generation taxoids: preparation, in vitro and in vivo evaluation. J. Control. Release. 2015;208:67–75. doi: 10.1016/j.jconrel.2015.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiao K, Luo J, Fowler WL, et al. A self-assembling nanoparticle for paclitaxel delivery in ovarian cancer. Biomaterials. 2009;30(30):6006–6016. doi: 10.1016/j.biomaterials.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davis ME, Chen Z, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat. Rev. Drug Discov. 2008;7(9):771–782. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- 24.Xu S, Olenyuk BZ, Okamoto CT, Hamm-Alvarez SF. Targeting receptor-mediated endocytotic pathways with nanoparticles: rationale and advances. Adv. Drug Deliv. Rev. 2013;65(1):121–138. doi: 10.1016/j.addr.2012.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Markman JL, Rekechenetskiy A, Holler E, Ljubimova JY. Nanomedicine therapeutic approaches to overcome cancer drug resistance. Adv. Drug Deliv. Rev. 2013;65(13–14):1866–1879. doi: 10.1016/j.addr.2013.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kamaly N, Xiao Z, Valencia PM, Radovic-Moreno AF, Farokhzad OC. Targeted polymeric therapeutic nanoparticles: design, development and clinical translation. Chem. Soc. Rev. 2012;41(7):2971–3010. doi: 10.1039/c2cs15344k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iyer AK, Khaled G, Fang J, Maeda H. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Disc. Today Targets. 2006;11(17–18):812–818. doi: 10.1016/j.drudis.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 28.Luo J, Xiao K, Li Y, et al. Well-defined, size-tunable, multifunctional micelles for efficient paclitaxel delivery for cancer treatment. Bioconjugate Chem. 2010;21(7):1216–1224. doi: 10.1021/bc1000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiao K, Luo J, Li Y, Lee JS, Fung G, Lam KS. PEG-oligocholic acid telodendrimer micelles for the targeted delivery of doxorubicin to B-cell lymphoma. J. Control. Release. 2011;155(2):272–281. doi: 10.1016/j.jconrel.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kato J, Li Y, Xiao K, et al. Disulfide cross-linked micelles for the targeted delivery of vincristine to B-cell lymphoma. Mol. Pharm. 2012;9(6):1727–1735. doi: 10.1021/mp300128b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin TY, Zhang H, Luo J, et al. Multifunctional targeting micelle nanocarriers with both imaging and therapeutic potential for bladder cancer. Int. J. Nanomed. 2012;7:2793–2804. doi: 10.2147/IJN.S27734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang H, Li Y, Lin TY, et al. Nanomicelle formulation modifies the pharmacokinetic profiles and cardiac toxicity of daunorubicin. Nanomedicine. 2014;9(12):1807–1820. doi: 10.2217/nnm.14.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiao K, Li Y-P, Wang C, et al. Disulfide cross-linked micelles of novel HDAC inhibitor thailandepsin A for the treatment of breast cancer. Biomaterials. 2015;67:183–193. doi: 10.1016/j.biomaterials.2015.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin TY, Li YP, Zhang HY, et al. Tumor-targeting multifunctional micelles for imaging and chemotherapy of advanced bladder cancer. Nanomedicine. 2013;8(8):1239–1251. doi: 10.2217/nnm.12.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shi C, Guo D, Xiao K, Wang X, Wang L, Luo J. A drug-specific nanocarrier design for efficient anticancer therapy. Nat. Commun. 2015;6:7449. doi: 10.1038/ncomms8449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Y, Lin T-Y, Luo Y, et al. A smart and versatile theranostic nanomedicine platform based on nanoporphyrin. Nat. Commun. 2014;5:4712. doi: 10.1038/ncomms5712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Y, Xiao K, Luo J, Lee J, Pan S, Lam KS. A novel size-tunable nanocarrier system for targeted anticancer drug delivery. J. Control. Release. 2010;144(3):314–323. doi: 10.1016/j.jconrel.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Y, Xiao K, Luo J, et al. Well-defined, reversible disulfide cross-linked micelles for on-demand paclitaxel delivery. Biomaterials. 2011;32(27):6633–6645. doi: 10.1016/j.biomaterials.2011.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiao K, Li Y, Lee JS, et al. “OA02” peptide facilitates the precise targeting of paclitaxel-loaded micellar nanoparticles to ovarian cancer in vivo . Cancer Res. 2012;72(8):2100–2110. doi: 10.1158/0008-5472.CAN-11-3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kenyon NJ, Bratt JM, Lee J, et al. Self-assembling nanoparticles containing dexamethasone as a novel therapy in allergic airways inflammation. PLoS ONE. 2013;8(10):e77730. doi: 10.1371/journal.pone.0077730. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.