Figure 3. Traditional inhibitors of proton motive force generation.
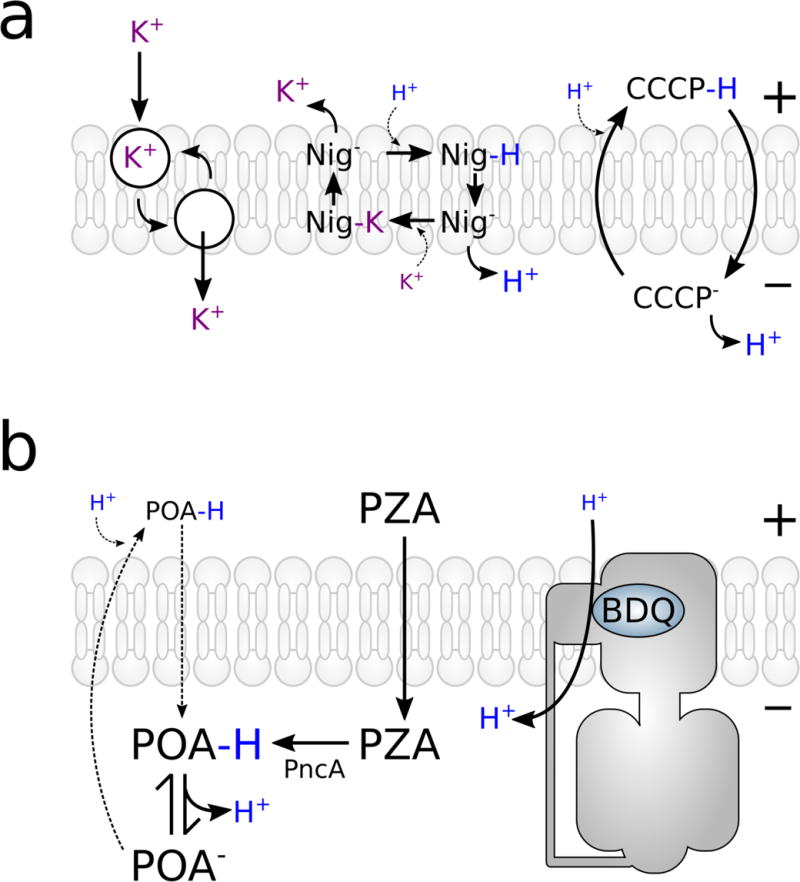
(A). Valinomycin is an ionophore, selective for potassium ions, which equilibrates the potassium gradient— dissipating the Δψ (electrogenic). Nigericin is a hydrophobic weak carboxylic acid, which can traverse the membrane as its either protonated acid or neutral salt. It dissipates chemical gradients (i.e. ΔpH) but maintains the charge (one positive charge exchanged for one positive charge—electroneutral). (3) Carbonyl cyanide m-chlorophenyl hydrazine (CCCP) is an electrogenic protonophore. CCCP− is driven to the periplasm by the Δψ, while CCCPH is driven to the cytoplasm by the ΔpH. It can equilibrate both Δψ and ΔpH. (B) Model for uncoupling by either pyrazinamide (PZA) or bedaquiline (BDQ). Left panel. PZA diffuses into the cell and is converted to pyrazinoic acid (POA) by PncA (pyrazinamidase). Anionic POA could effectively inhibit growth through anion accumulation in the neutral pH of the cytoplasm and/or efflux from the cells to become protonated in the acidic extracellular environment (POA−H). POA−H would then diffuse back into the cell driven by the ∆pH gradient and dissociate in the cytoplasm (neutral pH) leading to intracellular acidification and cell death. Right panel. In a typical mycobacterial cell, the majority of ATP synthesis is respiratory, driven by the pmf. The binding of BDQ to the c-ring most likely perturbs the a-c subunit interface, causing an uncontrolled proton leak uncoupled from ATP synthesis and resulting in a futile proton cycle. Compensation by the exchange of other cations (i.e. K+) would allow the process to remain electroneutral.
