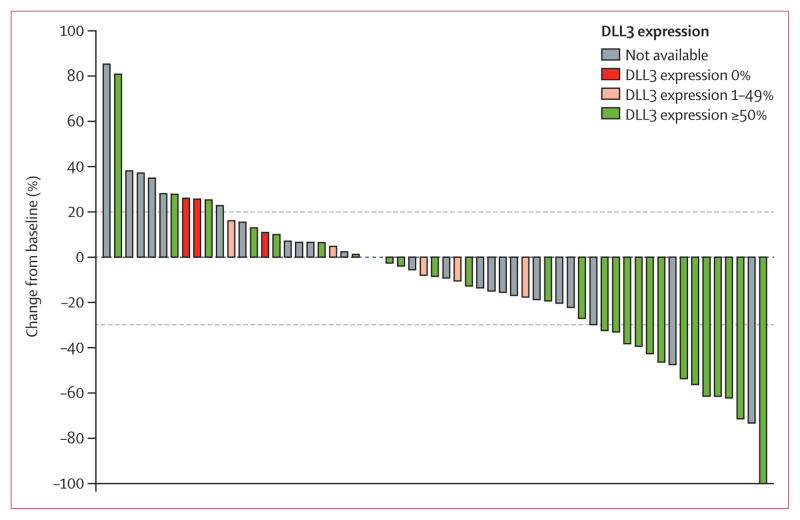
Figure 1. Waterfall plot showing best change in tumour burden from baseline at active treatment doses (n=60).
Investigator-assessed best change from baseline was the change in the sum of longest diameters of target lesions for patients treated with rovalpituzumab tesirine 0·2 mg/kg or 0·4 mg/kg every 3 weeks or 0·3 mg/kg or 0·4 mg/kg every 6 weeks. Grey dotted line at 20% indicates the threshold for progressive disease and the line at –30% the threshold for partial response. One patient did not have a measurable target lesion.