Figure 5. Slit/Robo signalling and MMP2 control asymmetry of L/R phrenic nerves.
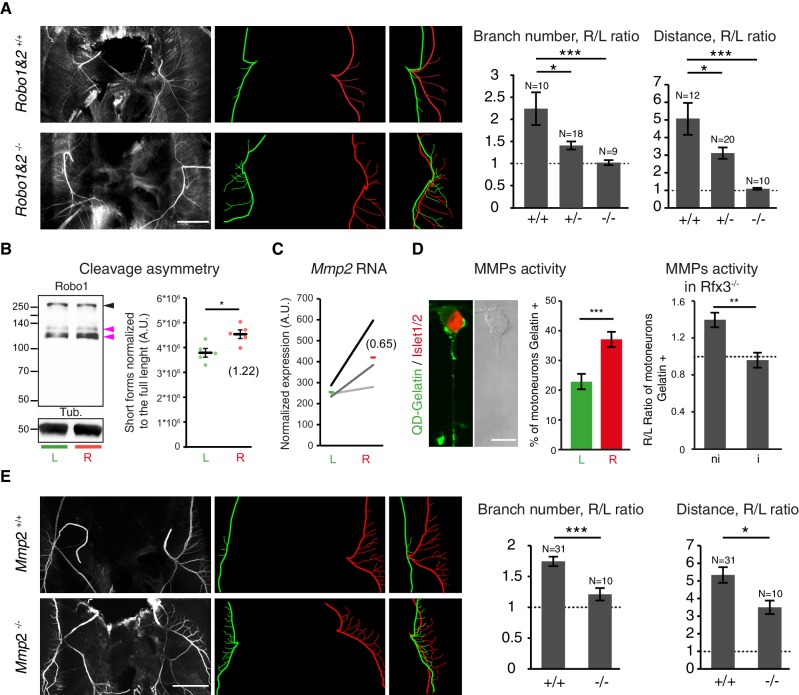
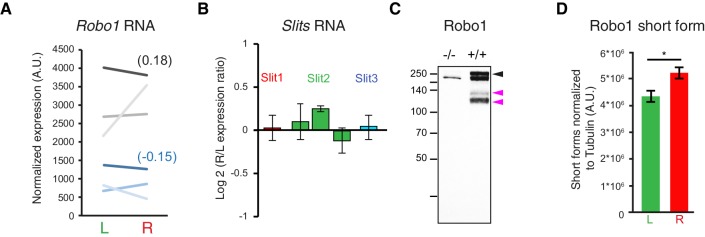
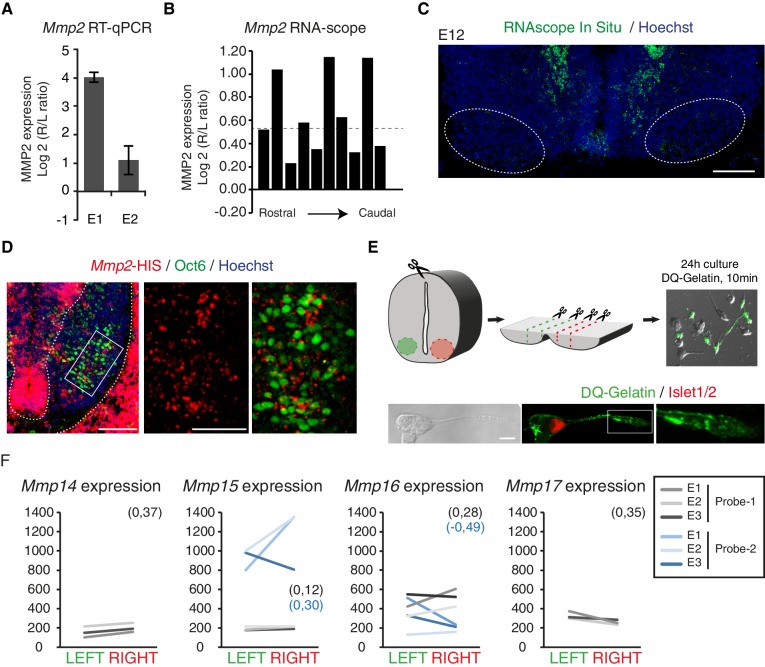
(A) NF staining of E14.5 diaphragm from Robo1+/+ and Robo2+/+ and Robo1–/– and Robo2–/–- embryos, left and right primary branches are pseudocolored in green and red,respectively, and superimposed to show the lack of asymmetry in the Robo1 and 2–/– embryos. Histogram showing the branch number and the defasciculation distance in Robo1+/+ and Robo2+/+, Robo1+/– and Robo2+/– and Robo1–/– and Robo2–/– embryos (R/L branch ratio: Robo1+/+ and Robo2+/+ 2.30 ± 0.37, versus Robo1–/– and Robo2–/– 1.06 ± 0.06; p=0.00048; R/L distance ratio: Robo1+/+ and Robo2+/+ 4.99 ± 0.89, versus Robo1–/– and Robo2–/– 1.05 ± 0.07; p=3E-6, Mann-Whitney). (B) Immunodetection of Robo1 and loading control (Tub) in left and right HB9::GFP ventral cervical spinal cord and distribution of the relative amount of the two shorter forms (pink arrowheads) to the full-length form (black arrowhead). The graph shows the normalized left and right values obtained for the five western-blots (dots, 6–8 embryos per sample) and the mean ± SEM (R versus L: p=0.01587, Wilcoxon singed rank); average fold-change is shown in brackets (1.22 ± 0.10). Normalization between lines was done on the Robo1 long form. (C) Ladder graph showing the left and right expression of Mmp2 detected by microarray in three embryos. Average Log2(R/L ratio) shown in brackets. (D) Photomicrograph of cultured ventral cervical spinal cord motoneuron. The combination of in situ zymmography with DQ-Gelatin and Islet1/2 staining enables the identification of motoneuron with MMP gelatinase activity. Histogram showing the amount of motoneuron with gelatinase activity in left and right samples (left 23.37% ± 2.7, N = 792 versus right 37.94% ± 2.1, N = 797; p=0.00109, Mann-Whitney). Histogram showing the gelatinase activity measured in cultures from Rfx3–/– embryos with symmetric lungs (Iso) and in cultures from Rfx3+/+, Rfx3+/– embryos (Rfx3 wt: — 1.4 ± 0.08; Rfx3 iso — 0.96 ± 0.08, p=0.0013, Mann-Whitney). (E) NF staining of E14.5 diaphragms from wild-type and Mmp2–/– embryos. Left (green) and right (red) primary and secondary branch traces shown in the middle panel are superimposed in the right panel to compare the left and right patterns. Histograms showing the R/L ratios of branch number and defasciculation distances. Ratio of secondary branches: Mmp2+/+ and Mmp2–/+ 1.74 ± 0.07, versus Mmp2–/– 1.21 ± 0.10; p=0.00029; defasciculation distance: Mmp2+/+ and Mmp2–/+ 5.33 ± 0.44, versus Mmp2–/– 3.49 ± 0.38; p=0.022, Mann-Whitney. Scale bar: 200 μm (A,E), 10 μm (D). Numerical values used to generate the graphs are accessible in Figure 5—source data 1.
DOI: http://dx.doi.org/10.7554/eLife.18481.022