Figure 5.
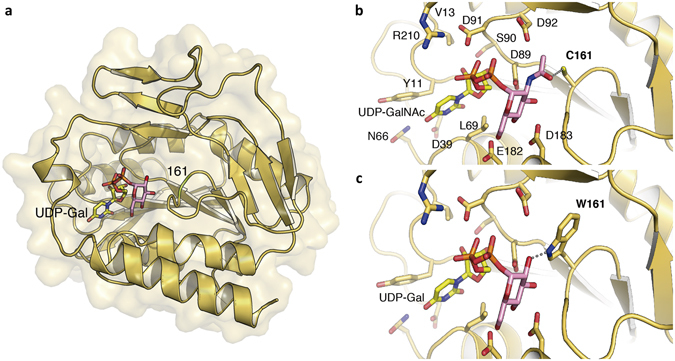
Three-Dimensional modeling of CpsK polymorphic variants. (a) The serotype 2 CpsK protein structure is depicted in yellow ribbon, and the position of amino acid residue 161 was colored in green and labeled. The docked substrate UDP-Gal is shown in sticks with the Gal moiety in pink. (b) Detailed view of the catalytic center of protein CpsK from serotype 1/2 with a cysteine residue at position 161 (C161) in complex with UDP-GalNAc as a substrate. Residues predicted to play a role in substrate binding and stabilization are depicted as capped sticks and labeled. Potential hydrogen bond between C161 and N-acetyl group of GalNAc is represented with a dashed grey line. (c) Same view as in panel a for the catalytic center of protein CpsK from serotype 2 with a tryptophan residue at position 161 (W161) in complex with UDP-Gal molecule as a substrate. Dashed grey line represents the potential hydrogen bond between W161 and the hydroxyl group of Gal.
