Figure 3.
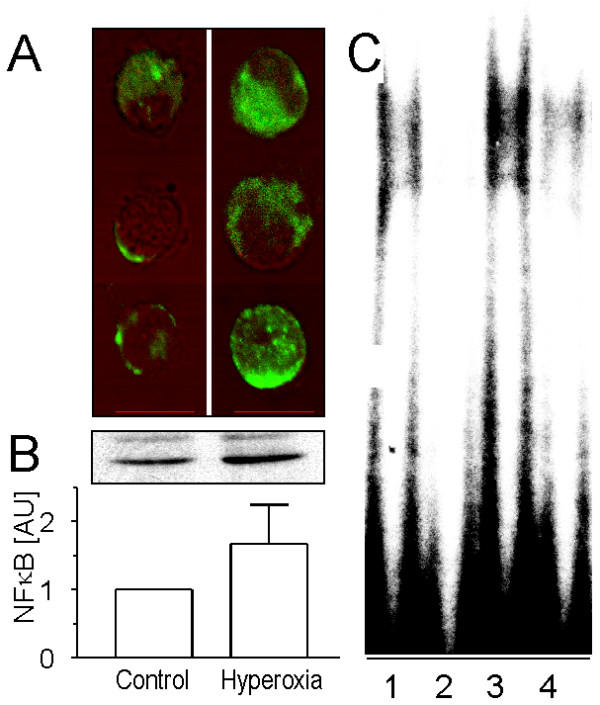
Activation of NF-κB in TIIcells and its enrichment in the nuclear protein fraction after hyperoxic treatment of rats. Freshly isolated TIIcells from normoxic (control) and hyperoxic rats were prepared for immunocytochemistry, SDS-PAGE and immunoblotting as described in Materials and Methods. A, activation of NF-κB was measured by immunocytochemistry using a monoclonal anti-NF-κB-antibody overlapping the nuclear localisation signal of the p65 subunit in the NF-κB heterodimer. Activated NF-κB in the nucleus and cytosol was quantified as described recently in detail [28]. The signal of activated NF-κB in the nucleus increased 4.7 fold in respose to hyperoxia (n = 12; p < 0.05). Bar: 10 μm. B, the nuclear protein fraction [52] of TIIcells was subjected to SDS-PAGE and immunoblotting. The p65 subunit of NF-κB was visualised using a rabbit polyclonal antibody, and its expression was densitometrically estimated. Values of n = 4 independent experiments are given as mean ± SD in arbitrary units (control = 1). C, Electrophoretic mobility shift assay for NF-κB in freshly isolated TIIcells from normoxic (lanes 1, 2) and hyperoxic (lanes 3, 4) rats. Signal competition upon addition of unlabelled oligonucleotide (lanes 2, 4).
