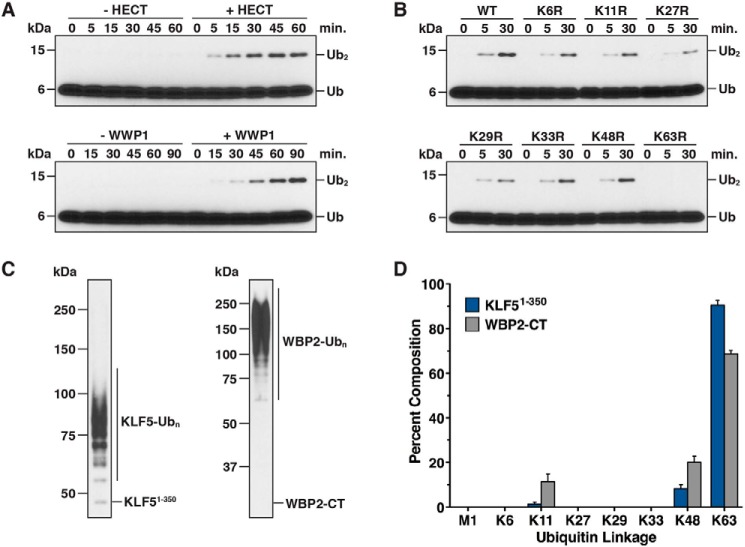
Figure 2.
Linkage specificity of Ub chains synthesized by WWP1. A, diubiquitin chain synthesis assays were conducted in the absence or presence of the WWP1 HECT domain (top panel) and in the absence or presence of full-length WWP1 (bottom panel). Reactions were quenched at the indicated times and resolved by Tris-Tricine SDS-PAGE, and the reaction products were analyzed by anti-ubiquitin immunoblotting. 2-fold higher concentrations of UbcH7 and WWP1 were required for robust detection of diubiquitin by full-length WWP1. B, diubiquitin chain synthesis assays were carried out with the WWP1 HECT domain as described in A, except the indicated lysine to arginine Ub mutant was substituted into the reaction. C, anti-His6 western blots showing the starting material used for quantification of Ub chain linkages by mass spectrometry. Ubiquitinated KLF51–350 and WBP2-CT substrates were purified from all other reaction components under denaturing conditions. D, quantification of Ub chain linkages synthesized by WWP1 using the ubiquitinated material shown in C. Lys-63, Lys-48, and Lys-11 linkages were the only linkages detected (see “Experimental procedures” for details). Data are represented as a percentage of the sum of all linkages detected. Error bars represent triplicate measurements (± S.D. of the mean).