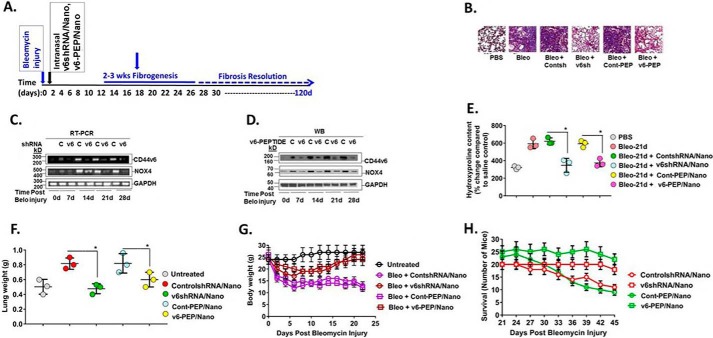
Figure 13.
In vivo targeting of CD44v6 by genetic modification, and with the CD44v6-blocking v6-PEP. A, the CD44v6 shRNA/nanoparticle (pSico-CD44v6 shRNA/Tf-PEG-PEI plus FSP-1-Cre/Tf-PEG-PEI), control (Con) shRNA/nanoparticle (pSico-scrambled shRNA/Tf-PEG-PEI plus FSP-1-Cre/Tf-PEG-PEI), or V6-PEP/nanoparticle were administered every other day from day 2 to day 30 by intratracheal delivery to the lungs of young mice during the onset of inflammation by bleomycin injury and then left untreated for another 15 days (see “Experimental procedures” for a detailed description of the shRNA/nanoparticle preparation and delivery method). Lung tissue was harvested at the indicated times up to 45 days after injury. The time course of fibrosis induction, treatment schedule, and resolution of fibrosis are shown. Nanoparticle preparation and delivery method was validated by our group previously in an intestinal/colon cancer murine model (45, 153). B, fibrosis was assessed by Masson's trichrome blue staining for collagen in sections of lungs isolated at day 24. Fibroblasts were isolated from lungs at 0, 7, 14, 21, and 45 days after bleomycin treatment in mice treated intratracheally with or without CD44V6 shRNA/nanoparticle, control shRNA/nanoparticle, or V6-PEP/nanoparticle and cultured ex vivo. C, total RNA was isolated from these fibroblasts, and real-time PCR analyses were done for CD44v6, Nox4, and Gapdh mRNA expression. D, whole-cell lysates were prepared from the isolated fibroblasts from the indicated days after bleomycin injury. Immunoblotting analyses were done for CD44v6, Nox4, and GAPDH protein expressions. E, quantitative hydroxyproline collagen assays were done for tissue samples from lungs collected at 0, 7, 14, 21, and 45 days after bleomycin injury in mice treated with or without CD44V6 shRNA/nanoparticle or control shRNA/nanoparticle. F–H, lung weight (F), body weight (G), and survival of mice over 45 days (H) were measured for the indicated days after treatment. The data in the experiments (A–H) are representative of three sets of three independent experiments with 10 mice in each experiment. Statistical analysis was with ANOVA (± S.D.; n = 3; *, p ≤ 0.001).