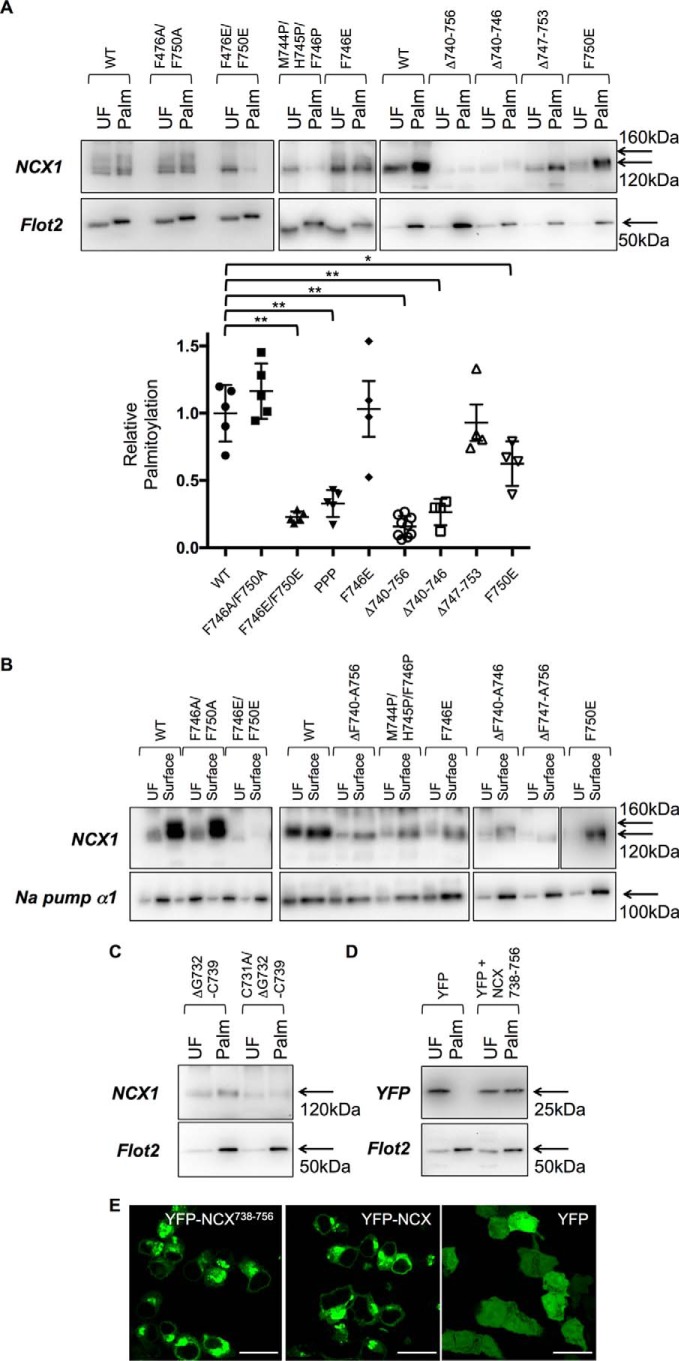
Figure 5.
Point mutations and palmitoylation of full-length NCX1. A, point mutations F746E/F750E, Δ740–756, Δ740–746, and M744P/H745P/F746P prevent palmitoylation of full-length NCX1, whereas F750E reduces full-length NCX1 palmitoylation, and Δ747–753 and F746E are without effect. B, of the mutations assessed in A, only F746E/F750E impedes progression of full-length NCX1 through the secretory pathway. C, NCX1 is palmitoylated at Cys-731 when the region usually on the C-terminal side of Cys-739 is instead positioned adjacent to Cys-731. D, C-terminal fusion of NCX1 residues 738–756 is sufficient to direct palmitoylation of YFP. E, C-terminal fusion of NCX1 residues 738–756 anchors YFP to intracellular membranes in a manner indistinguishable from YFP-NCX1. Scale bar, 20 μm. *, p < 0.05 versus WT; **, p < 0.01 versus WT, n = 5.