Figure 1.
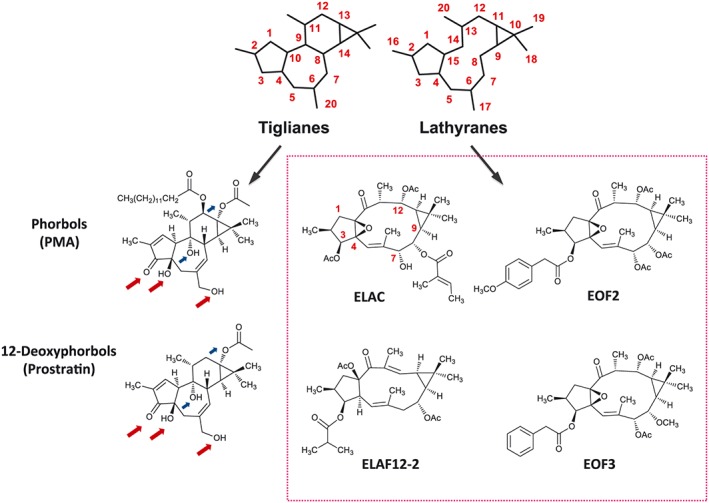
Schematic representations of the chemical skeletons of tiglianes and lathyranes, two classes of polycyclic diterpenes that are abundant in the latex of Euphorbia plants. Tiglianes like phorbols and 12‐deoxyphorbols – being PMA and prostratin the stereotypical members of these types, respectively – are well‐known PKC activators that promote the proliferation of NPC (Geribaldi‐Doldán et al., 2016). Red arrows pinpoint C3, C4 and C20‐bound oxygen residues, identical in PMA and prostratin, thought to be important for PKC recognition and activation. Blue arrows pinpoint other oxygen residues that might also be involved in PKC activation. The tumourigenicity of phorbols like PMA makes them unsuitable for clinical research, while less toxic molecules, such as prostratin, are more propitious pharmacological candidates. In the search for new molecules with clinical value, and given the basic structure similarity between tiglianes and lathyranes, we have tested whether lathyranes could also activate PKC and induce NPC proliferation; the four lathyranes tested are shown inside the box. Abbreviations: Ac, acetyl.
