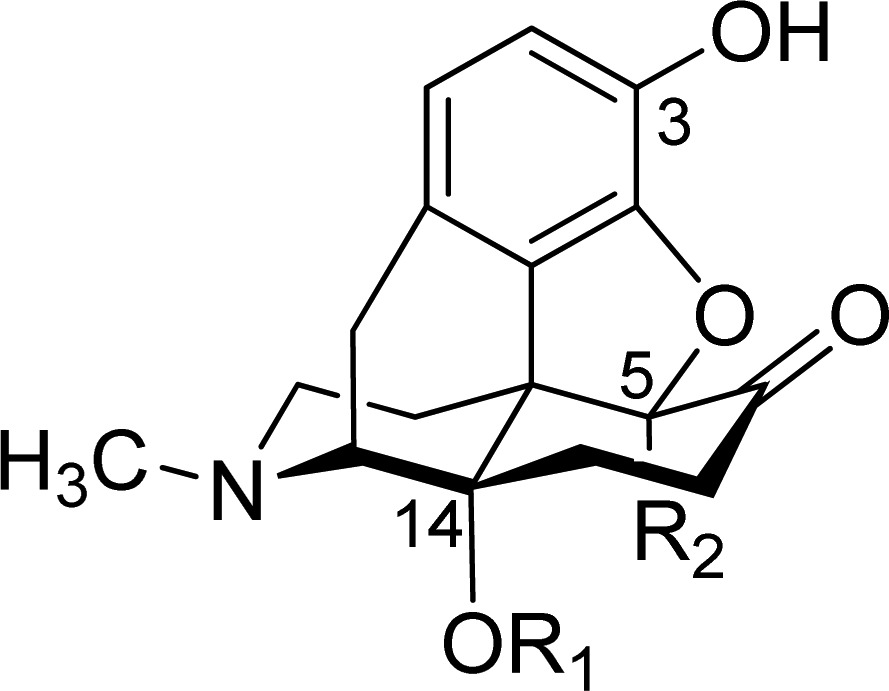
Table 1. Structures, Binding Affinities and Selectivities for the μ-OR, and Antinociceptive Potencies of Oxymorphone (1) and Investigated 14-Oxygenated N-Methylmorphinan-6-ones (2–6)a.
| in vitro μ-OR bindingc |
|||||
|---|---|---|---|---|---|
| ligand | R1, R2b | μ-OR affinity (Ki, nM) | μ-OR selectivity vs δ-OR | μ-OR selectivity vs κ-OR | antinociceptive potencyd |
| OM (1) | H, H | 0.97 | 83 | 63 | 13,e 18,f 10g |
| 14-OMO (2a) | Me, H | 0.10 | 48 | 102 | 810,e 300,f 126,g |
| 14-MM (2b) | Me, Me | 0.15 | 89 | 168 | 99,e 82,f 94g |
| 14-OEO (3a) | Et, H | 0.15 | 60 | 91 | 316e |
| 14-EM (3b) | Et, Me | 0.46 | 26 | 94 | 46e |
| 14-OBO (4a) | Bz, H | 0.12 | 18 | 10 | 697g |
| 14-BM (4b) | Bz, Me | 0.18 | 20 | 14 | 103g |
| PPOM (5) | PhPr, Me | 0.20 | 0.7 | 2 | 2500,e 24000,f 8500g |
| BOMO (6) | Me, Bz | 0.31 | 42 | 73 | 53,f 50g |
Bz, benzyl; Et, ethyl; Me, methyl; PhPr, phenylpropyl.
Determined by in vitro radioligand binding assays with rat brain membranes.
Relative to morphine, determined in mice after s.c. administration using the indicated tests.
Acetic acid-induced writhing test.
Tail-flick test.
Hot-plate test.
