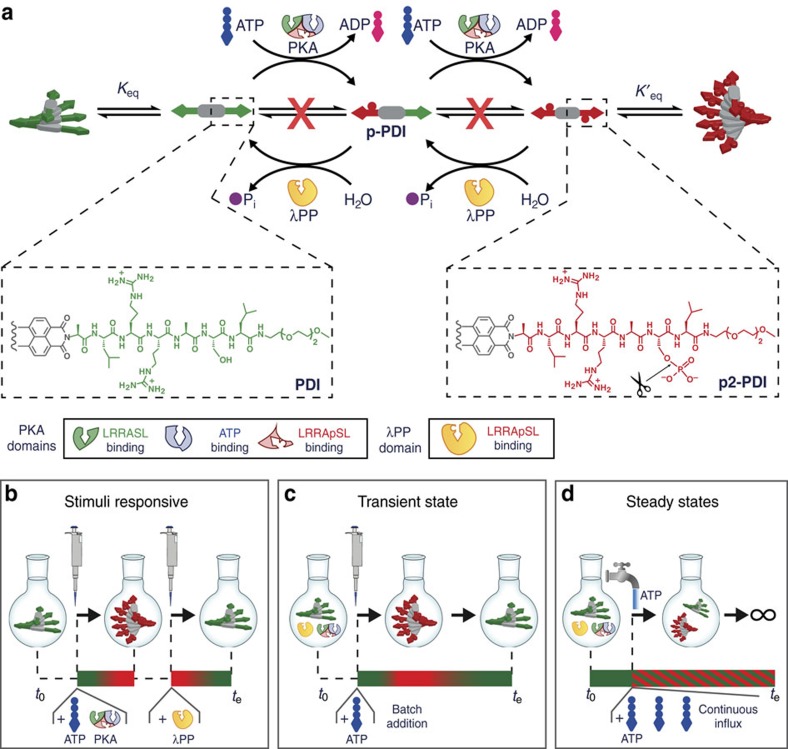
Figure 1. Enzyme-controlled supramolecular polymerization from stepwise to steady states.
(a) Peptide–perylenediimide derivative PDI (half is shown) is phosphorylated on the serine residue by protein kinase A (PKA) to give monophosphorylated p-PDI, and further diphosphorylated p2-PDI, fuelled by ATP to ADP hydrolysis (one eq. per phosphate introduced). Phosphate hydrolysis (scissors) by λ-protein phosphatase (λPP) yields inorganic phosphate Pi as waste. Both PDI and p2-PDI can self-assemble to form equilibrium supramolecular polymers. PKA has three binding sites: for ATP (blue), for the LRRASL peptide (green) and for LRRApSL (red). (b) Stimuli responsiveness: the addition of ATP and PKA to a solution of PDI results in p2-PDI and a consequent change of the supramolecular structure of the polymer. A second stimulus, that is, the addition of λPP, is needed to reset the polymer to its original nonphosphorylated state. (c) Transient state: a single input, that is, the addition of ATP to a solution of PDI, in the presence of PKA and λPP, leads to a transient change of the supramolecular structure and chirality of the polymer. (d) Supramolecular non-equilibrium steady states (NESS). The system is kept in a dissipative steady state by continuous influx of ATP. Depending on the level of the chemical fuel supplied different dissipative steady states can be accessed.