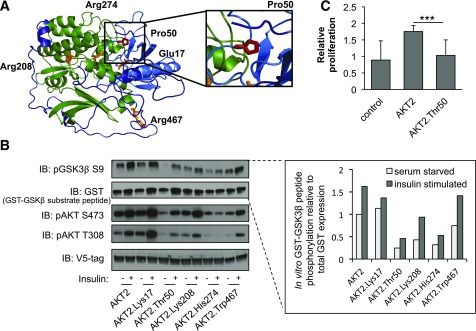
Figure 3.
Functional properties of AKT2-Thr50. A: Predicted protein structure of AKT2. Domain and variants are highlighted as in Fig. 2A. The relative spatial positioning of the AKT2-Pro50 residue is magnified within the inset. B: HeLa cells were infected with lentiviral V5-AKT2, V5-AKT2-Lys17, V5-AKT2-Thr50, V5-AKT2-Lys208, V5-AKT2-His274, or V5-AKT2-Trp467; starved for 18 h (white bar); and stimulated for 20 min with 100 nmol/L insulin (gray bar). V5-tagged AKT2 was isolated from cell lysates with anti-V5 agarose beads and incubated with GSK3β-GST peptide in an in vitro kinase assay. Quantification of phosphorylated substrate peptide (pGSK3β) relative to total peptide (GST-GSK3β) is shown at the inset. Immunoblots and quantification shown are representative of three independent replicates. Linear model statistical analyses across all three independent replicates are available in Supplementary Fig. 9. The in vitro kinase was immunoblotted (IB) with the indicated antibodies. C: HuH7 cells were infected with lentiviral V5-AKT2, V5-AKT2-Thr50, or control pLX304. At 72 h, relative cellular proliferation was determined with WST-1 assay of HuH7 cells. Error bars represent SD. ***P = 4.5 × 10−5.