Figure 3. Allosteric modulators can differentially modulate receptor populations based on their dimerization.
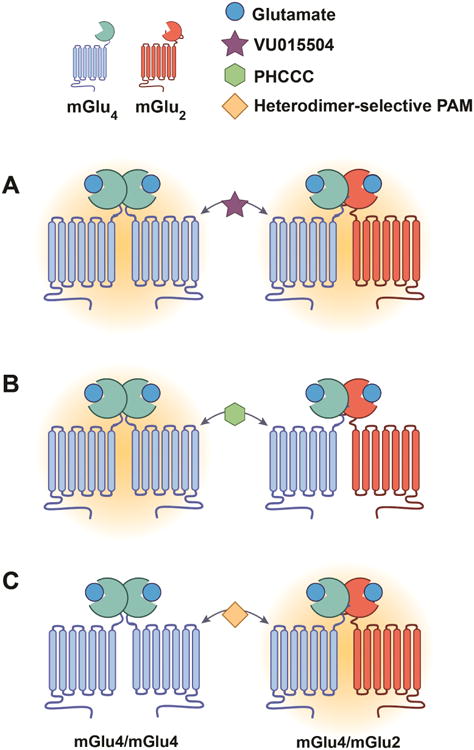
Many GPCRs can form oligomers either as homodimers (containing two receptors of the same subtype) or heterodimers (containing two different receptor subtypes). As depicted above using mGlu4 and mGlu2 receptors as an example, some PAMs (such as VU0155041) are active at both mGlu4/mGlu4 homodimer and mGlu4/mGlu2 heterodimer complexes (A; Potentiation of receptor activity is depicted by orange shading). Other PAMs (such as PHCCC) selectively activate mGlu4 homodimers but are inactive at mGlu4/mGlu2 heterodimers (B). Furthermore, while no examples have been described to date, it is possible that other classes of PAMs could be active at the heterodimer, but inactive at the homodimer (C). These novel tools that can distinguish between receptor complexes will shed light onto the physiological significance of homodimer and heterodimer complexes and could provide therapeutic benefits through incredibly precise modulation of circuitry that targets not only a given receptor subtype, but can target specific receptor complexes.
