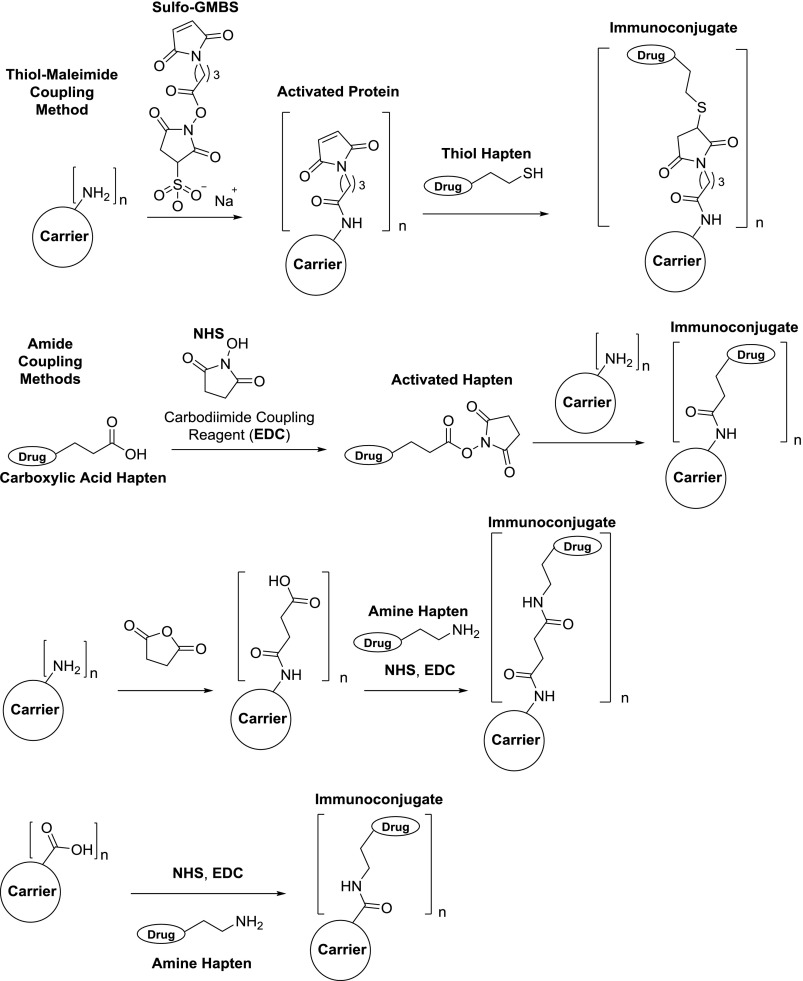
Fig. 8.
Commonly used bioconjugation methods for generating drug hapten–protein immunoconjugates. (Top) Surface lysine residues on the carrier protein are used for loading of maleimides. Thiol haptens can perform Michael addition to the maleimides to generate the conjugate. (Upper middle) N-hydroxysuccinimide (NHS) esters are formed from carboxylic acid haptens using a carbodiimide-coupling reagent, e.g., 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC). The resulting esters can then be reacted with surface protein lysines to form the conjugate. (Bottom middle) Surface protein lysines are modified with succinic anhydride, which allows for amide coupling to an amino-terminal hapten. (Bottom) Instead of lysines, carboxyl-containing amino acids on the carrier (aspartic and glutamic acids) can be coupled directly to amino-terminal haptens to generate immunoconjugates. Typical hapten loading onto carrier proteins: n = 5–30.