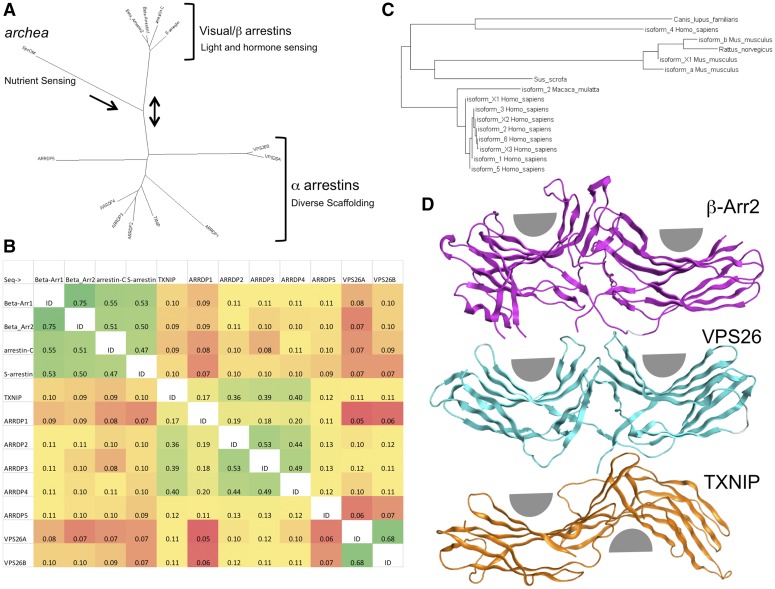
Fig. 1.
Evolutionary relationship of proteins sharing the common arrestin fold. (A) Radial phylogram showing the relation of all mammalian arrestins to the archaea arrestin-like molecule SpoOM. It also shows the relation to other mammalian arrestin family members, including the VPS and TXNIP proteins, which suggest that arrestins evolved from a mechanism to regulate environmental sensing toward more complicated signaling. NCBI BLAST was queried using the human β-arrestin2 full-length sequence. Psi-BLAST was used to search the RefSeq database. The sequence analysis was performed using Fitch phylogenetic tree analysis implemented in BioEdit 7.2.5 and visualized using Dendroscope 3.2.4. This graph was inspired from (Alvarez, 2008; Aubry et al., 2009). (B) Sequence identity matrix indicating the overall similarity of human arrestins. The analysis was performed using BLOSOM62 implemented in BioEdit 7.2.5. Cells were colored as a heat map using EXCEL according to percent similarity, with green being most similar and red being divergent. (C) Rectangular phylogram showing the relation of mammalian β-arrestin2 isoforms across several species. It demonstrates an expansion of diversity in humans and mice that may be indicative of the need to regulate complex hormone signaling and olfaction. The sequence analysis was performed using Fitch phylogenetic tree analysis implemented in BioEdit 7.2.5 and visualized using Dendroscope 3.2.4. (D) Structural comparison of β-arrestin, VPS26, and TXNIP showing different rotations between the amino and carboxy arrestin domains. VPS26 β-baskets are nearly symetirical and on the same plane, and β-arrestins show a compaction of the amino-terminal domain and a slight rotation between domains, whereas TXNIP has an almost 180° degree inversion of the relative position of the β-baskets. Structural PDB files (2WTR, 4P2A, and 4LL4) were superposed using only the amino-terminal domain to highlight the relative differences in the carboxy domain. Gray half circles were added to show the relative positions of the concave portion of the two β sandwiches per molecule. Analysis and visualization were performed using MOE 2014.09.