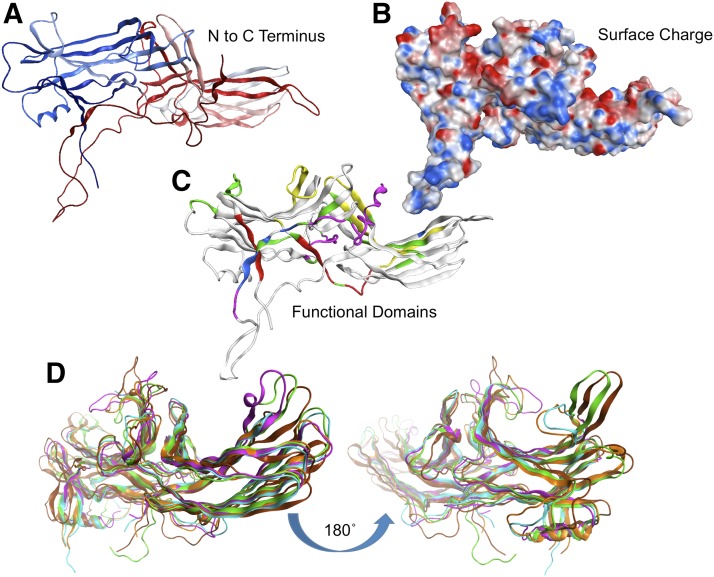
Fig. 3.
Visual/β-arrestin topologic structural analysis showing the overall tertiary fold of arrestins, the charge distribution surface, and the major functional and interaction domains. (A) Ribbon diagram indicating arrestin folding from N terminus (blue) to C terminus (red). (B) Surface diagram indicating the positive (red) and negative (blue) charge regions. (C) Functional domain diagram showing areas of functional importance from X-ray and mutagenesis studies. Domains are colored such that red regions are involved in receptor binding, green regions are involved in oligomerization, blue are important in arrestin activiation, and yellow regions interact with microtubules. Analysis and visualization were performed using MOE 2014.0. (D) Comparison of multiple X-ray crystal structures of β-arrestins shows plasticity and signaling diversity. The images show large conformational rearrangements localized to the outer loops, and the hinge domain proximal to the N-terminal domain. Note the disordered and unresolved loops present in the bottom of each image corresponding to the beginning of the C-terminal arm containing the CBD, Motif V, and RxR motifs. Topologic flexible regions of arrestin from PDB files 2WTR, 3GD1, 3GC3, 1AYR.A, and 1AYR.B. The two images are rotated 180° to each other to show the amino or carboxy domains. Structures were aligned and superposed using all carbon α atoms. Each chain has a unique color. Analysis and visualization were performed using MOE 2014.09.