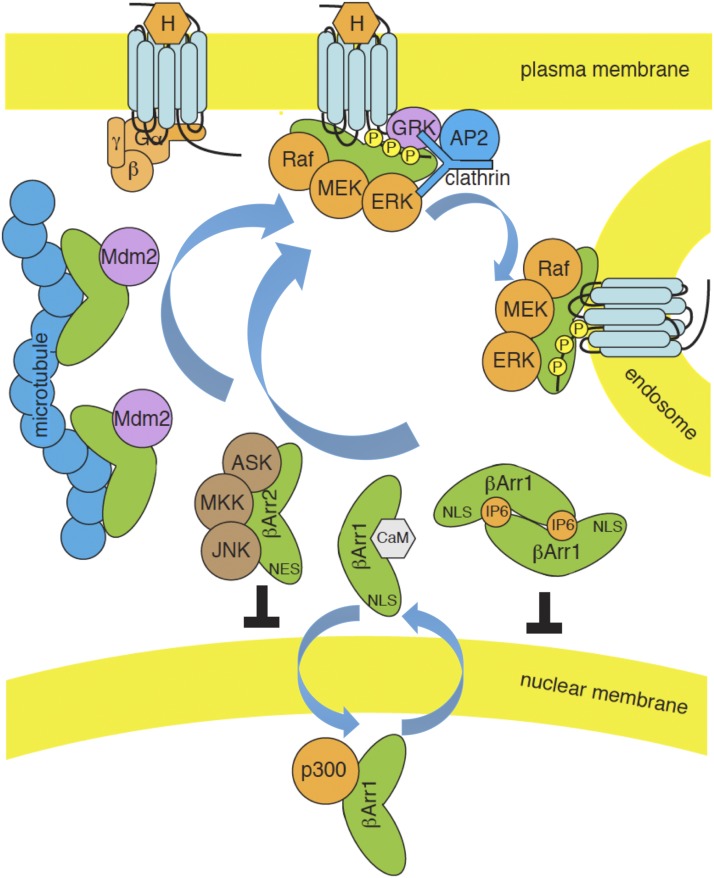
Fig. 4.
Dynamic regulation of functionally discrete arrestin pools. Visual/β-arrestins exist in equilibrium between a large intracellular pool, where they are either freely cytosolic or associated with low-affinity microtubule binding sites, and a small pool bound with high affinity to activated GPCRs. Cytosolic, microtubule-bound, and GPCR-bound arrestins adopt different conformations, such that some cargos preferentially associate with free arrestins, e.g., Ca2+-calmodulin and components of the ASK1/MKK4/JNK3 cascade, others prefer microtubule-bound arrestin, e.g., Mdm2, whereas still others preferentially associate with GPCR-bound arrestin, e.g., Raf-MEK-ERK1/2. Upon ligand (H) binding, GRK-phosphorylated GPCRs recruit β-arrestins from the cytosolic and microtubule-bound pools to the plasma membrane, where they can engage clathrin and AP2, leading to receptor endocytosis. Assembly of multiprotein signaling complexes on the GPCR-arrestin scaffold leads to spatially contrained pools of activated cargo, e.g., ERK1/2. Although β-arrestin2 (βArr2) is excluded from the cell nucleus by its NES, β-arrestin1 is in equilibrium between cytosolic and nuclear pools. IP6 binding promotes β-arrestin1 self-association, which, like microtubule binding, sequesters it from the nucleus and restrains interactions with transcriptional regulatory proteins, e.g., histone acetyltransferase p300.