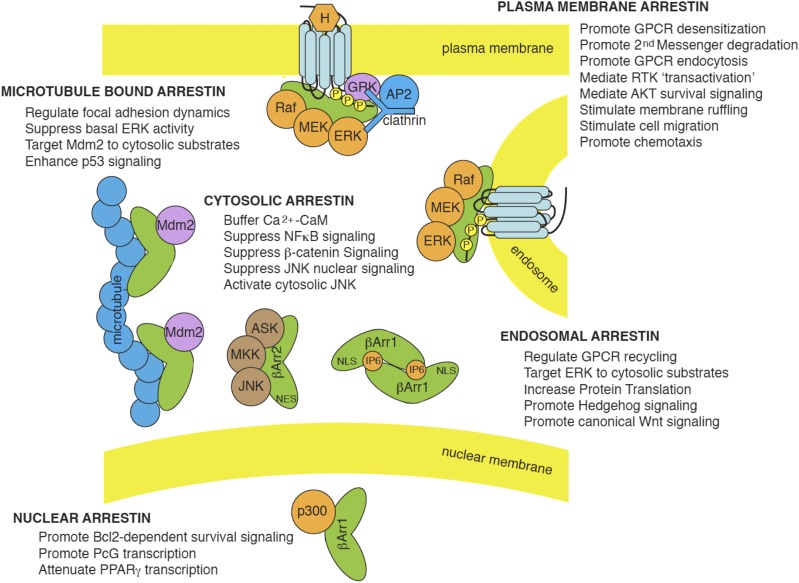
Fig. 5.
Diverse cellular functions of arrestin scaffolds. By associating with different cargos in different subcellular locations, visual/β-arrestins regulate multiple signaling networks. In quiescent cells, free cytosolic and microtubule-bound arrestins dampen basal pathway activity by sequestering signaling pathway intermediates away from their site of activation/action. Free arrestins can buffer cytosolic Ca2+–CaM concentration, suppress NFκB signaling by sequestering IκB kinases, and tonically inhibit β-catenin signaling by promoting GSK3β-dependent β-catenin phosphorylation and degradation. They also keep proapoptotic JNK kinases away from their nuclear substrates, whereas, in the case of β-arrestin2, they promote activation of cytosolic JNK. Microtubule-bound arrestins sequester inactive ERK1/2 away from the plasma membrane, dampening basal pathway activity, while directing Mdm2 toward cytoskeletal substrates. In some settings, this has the effect of increasing proapoptotic p53 signaling by preventing p53 ubiquitination and degradation. Microtubule-bound arrestins also regulate cell adhesion by binding to regulators of focal adhesions such as Src, ERK1/2, and JNK. Once recruited to plasma membrane-bound GPCRs, arrestins promote GPCR desensitization, support clathrin-dependent endocytosis, and accelerate second-messenger degradation by recruiting cAMP phosphodiesterases and diacylglycerol kinase. At the membrane they also stimulate cell proliferation by promoting Src-dependent transactivation of EGF receptor tyrosine kinases (RTKs) and promote cell survival by activating AKT. Through their interactions with numerous regulators of actin cytoskeletal dynamics, arrestin stimulate membrane ruffling, cell migration, and chemotaxis. Several β-arrestin cargos, e.g., ERK1/2, continue to signal from endosomal GPCR–arrestin signalsome complexes, where they regulate aspects of GPCR trafficking and recycling and preferentially phosphorylate cytosolic ERK substrates, leading to increased protein translation. β-Arrestins also stimulate canonical Wnt signaling by engaging Dsh and inhibiting GSK3β to stabilize β-catenin, and promote hedgehog signaling by internalizing and targeting smoothened to primary cilia. Within the nucleus, β-arrestin1 interacts with a number of transcription factors to either increase or tonically inhibit transcription.