Figure 2.
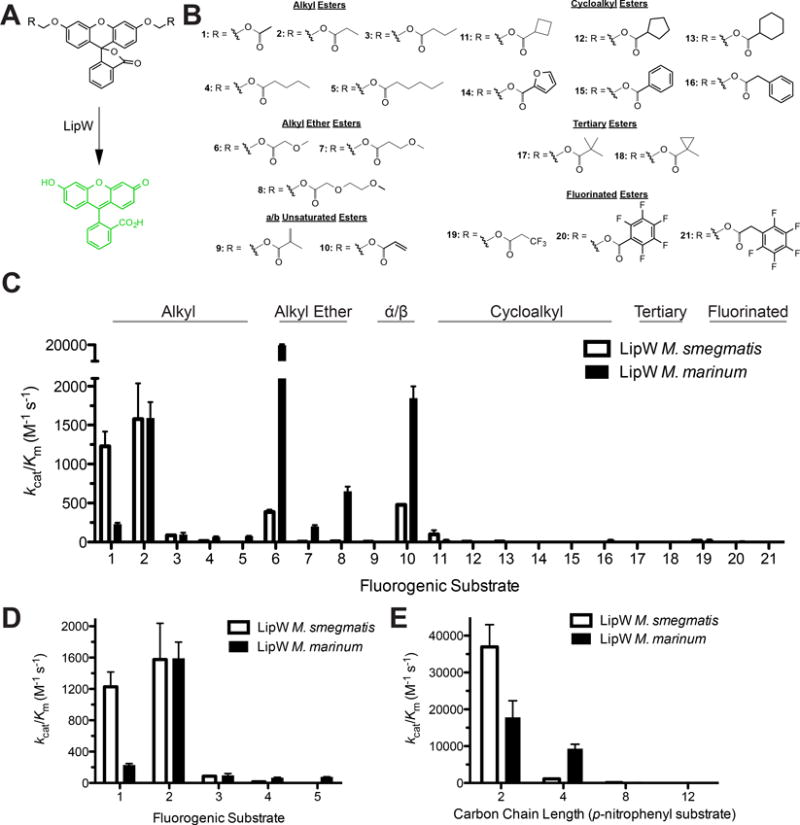
Comparative kinetics of LipW homologues using a fluorogenic substrate library. A) Activation of fluorogenic substrates by LipW. Hydrolysis of the ester bond on the diacyloxymethyl ether fluorescein substrates by LipW converts the fluorescein core from the nonfluorescent lactone form to the highly fluorescent quinoid form. The rate of fluorophore activation is measured at a range of substrate concentrations to determine the kinetic constants for fluorophore activation. B) Each of the substrates is composed of diacyloxymethyl ether fluorescein with varying R-groups. The differing R-groups have been organized into classes based on chemical functionality. All of the substrates were synthesized as described previously.38;42;51 C) Global comparison of the catalytic specificity (kcat/KM) of LipW against each of the 21 substrates (structures and numbering given in Fig. 3B) with ester classes labeled. D) Substrate specificity of LipW against alkyl ester substrates, illustrating the substrate selectivity based on the carbon chain length. E) Catalytic efficiency of LipW against p-nitrophenyl substrates (p-nitrophenyl acetate (2), p-nitrophenyl butyrate (4), p-nitrophenyl octanoate (8), and p-nitrophenyl laurate (12)). Catalytic efficiency values (kcat/Km) are given ± SE. Detailed kinetic results for each substrate are provided in Supplemental Tables 1 and 2 with representative kinetic plots shown in Supplemental Figure 2.
