Figure 3.
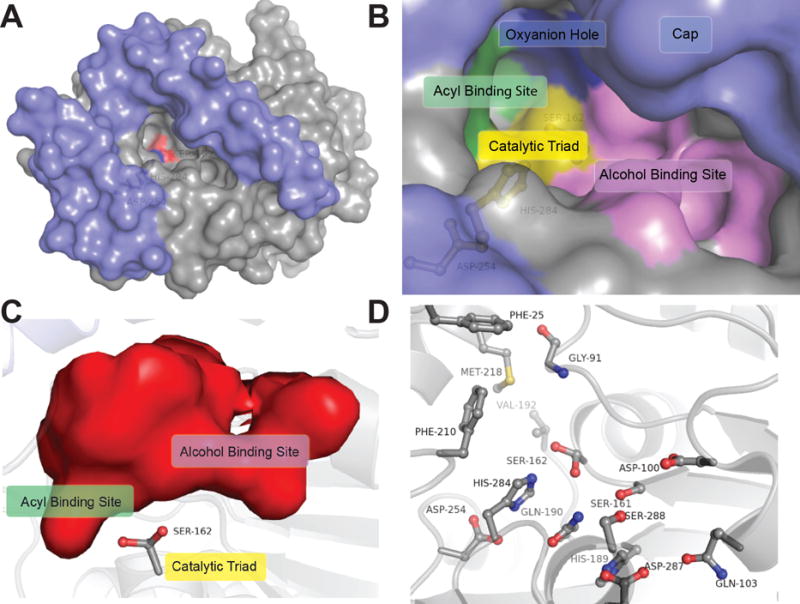
Binding pocket architecture of LipW. A) Cap domain (blue) and binding pocket surface of LipW (grey) with the nucleophilic serine (Ser162) in red and catalytic histidine (His284) in blue. B) Surface representation of the binding pocket and active site of LipW. Individual subsections within the LipW binding pocket are labeled. C) Interior surface of the LipW binding pocket with Ser162 labeled. Binding pocket rotated 90° clockwise from part B and oriented from the interior of the protein. Subsections labeled as in part B. D) Binding pocket and catalytic residues substituted with alanine in LipW. Each of the residues shown in ball and stick was individually substituted with alanine and the relative contribution of each side-chain to the catalytic activity and thermal stability determined (Figure 4).
