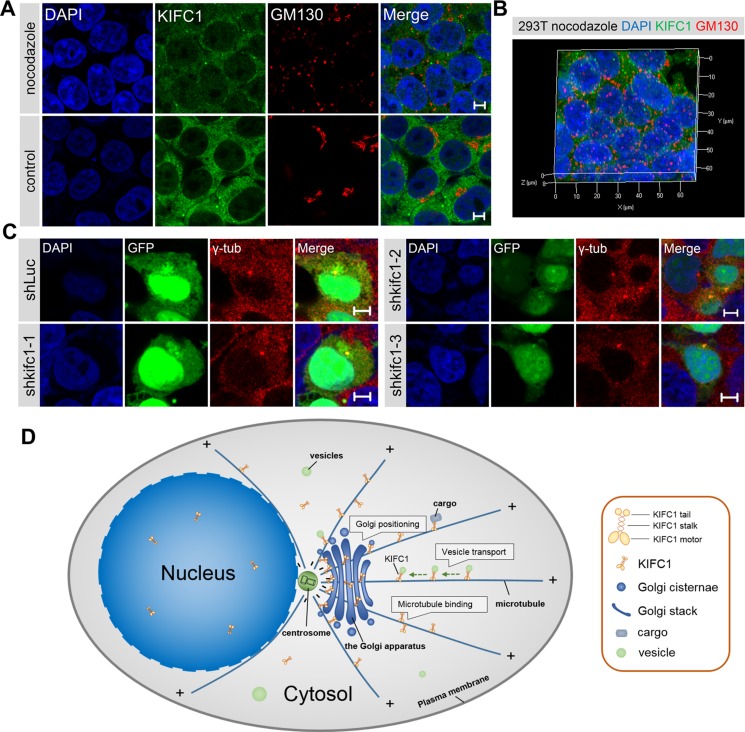
Figure 6. The interactions between kinesin-14 KIFC1 and microtubule networks in the Golgi positioning.
(A) Representative immunoflurescence images of KIFC1 and the Golgi marker GM130 in HEK293T cells in the control cells and in the nocodazole treatment group (10 μM/3 h). DAPI (blue), KIFC1 (green), GM130 (red). Scale bars, 5 μm. (B) Three dimensional images of the fluorescence signal of nucleus (blue), KIFC1 (green) and the Golgi marker GM130 (red) in HEK293T cells after incubation with nocodazole (10 μM/3 h). (C) Representative images of the localization of γ-tubulin in HEK293T cells after kifc1 knockdown for 72 hr. DAPI (blue), GFP (green), γ-tubulin (red). GFP is the fluorescence protein in shRNA plasmid, and is used to indicate the transfection of shRNA plasmid. (D) The model for the functions of the minus end-directed kinesin-14 KIFC1 in the Golgi positioning and architectural maintenance in non-polarized mammalian cells. The microtubules are organized at the MTOC near the cell center and form characteristic radial arrays extending toward the cell periphery in non-polarized mammalian cells. In the cytoplasm, kinesin-14 KIFC1 proteins traffic along the microtubules, from the plus ends to the minus ends, and accumulate at the pericentrosomal region. During vesicle transport, kinesin-14 KIFC1 transports the Golgi-associated vesicles to the minus ends near the MTOC. The dynamic movements of kineisn-14 KIFC1 motors along the microtubules are fulfilled by the step-by-step walking of the motor domain using ATP-hydrolyzing energy and the cargo-binding affinity of the tail domain. Moreover, at the minus end of the microtubules around the MTOC, the kinesin-14 KIFC1 protein can bind to the Golgi apparatus via its motor domain and can also statically bind to the microtubules via its tail domain, thus severing as a static crosslinker between the Golgi apparatus and the microtubules around the pericentrosomal region near the nucleus.