Abstract
Background
Counseling patients about the risk of future pregnancy on hormone receptor-positive breast cancer outcomes is difficult with minimal data and understanding of pregnancy on the breast environment.
Patients and Methods
This retrospective analysis included 32 pre-menopausal women with a diagnosis of estrogen receptor-positive breast cancer between 2000 and 2010 and subsequent pregnancy within 5 years. The control cohort included 29 women matched for age and stage of breast cancer but did not become pregnant.
Results
There was no statistically significant difference in age, diagnosis, stage, grade or HER2 status between groups. Nineteen (63%) women in the pregnancy cohort received endocrine therapy and 23 (82%) in the control cohort (P=0.25). The mean (range) length of endocrine therapy in the control cohort was 42.3 (0–120) months, compared to the pregnancy cohort, 20.9 (0–72) months (P=0.008). Four women (14%) in the control cohort had a breast cancer recurrence compared to eight women (26%) in the pregnancy cohort, (P=0.34). The five-year disease free survival was 92% (95% CI 81% to 100%) in the control cohort compared to 84% (95% CI 72% to 97%) in the pregnancy cohort, which was not statistically significant (P=0.69).
Conclusions
This study did not demonstrate poorer DFS in pre-menopausal women with estrogen receptor-positive breast cancer that became pregnant within five years of diagnosis. It is unique because all included patients had estrogen receptor-positive disease and were offered adjuvant hormone therapy. Further prospective investigation will be beneficial to patients and physicians as they discuss pregnancy as a key survivorship issue.
MicroAbstract
Pregnancy after diagnosis of breast cancer is an important survivorship issue. We conducted a retrospective analysis including 32 pre-menopausal women with a diagnosis of estrogen receptor-positive breast cancer and subsequent pregnancy and an age- and stage-matched control cohort without subsequent pregnancy. This study did not demonstrate poorer DFS in women that became pregnant within five years of their cancer diagnosis.
Introduction
Breast cancer is the most commonly diagnosed malignancy in women between the ages of 30 and 45 and this age group accounts for 10.8% of newly diagnosed breast cancer cases each year in the United States.1 With a trend in women delaying pregnancy and childbirth until later in life, fertility preservation after a diagnosis of breast cancer and future pregnancy is a growing concern.2 While there have been great advancements in fertility preservation for cancer patients, many women and their physicians are hesitant to consider or recommend pregnancy to women with a history of hormone receptor-positive breast cancer and unfortunately, many patients are recommended against future pregnancy.3 While the potential for negative consequences due to the increased hormonal milieu associated with pregnancy is of high theoretical concern to physicians, there is no data that suggests pregnancy after a diagnosis of breast cancer is associated with higher rate of recurrence or death. Furthermore, there is limited data on the risk of breast cancer recurrence after pregnancy since tamoxifen has become the standard of care for adjuvant therapy in hormone receptor positive breast cancer and significantly improved outcomes. We investigated the breast cancer outcomes of premenopausal women diagnosed with hormone receptor-positive breast cancer at our cancer center during the tamoxifen era and subsequently became pregnant within five years of their breast cancer diagnosis.
Patients and Methods
Patients included in the pregnancy cohort were pre-menopausal females, ages 18 years or older with a diagnosis of breast cancer including ductal carcinoma in situ, invasive ductal carcinoma or invasive lobular carcinoma that was stage 0 to stage IIIA (Tis-T3, N0–N2). The tumor must have been estrogen-receptor positive (>1%). All included women needed to have been considered for adjuvant tamoxifen therapy. Women in the pregnancy cohort must have achieved a pregnancy (including miscarriage) within five years of diagnosis of breast cancer.
Patients in the control (no pregnancy) cohort were also pre-menopausal females, ages 18 years or older also diagnosed with stage 0 to stage IIIA, estrogen-receptor positive breast cancer. Using the Northwestern Medicine Enterprise Data Warehouse, the control group was an age- and stage-matched cohort to the pregnancy cohort. However, these patients did not achieve a pregnancy within five years of a diagnosis of breast cancer. All included women had been considered for adjuvant tamoxifen therapy.
A query was conducted in the Northwestern Medicine Enterprise Data Warehouse to identify female patients 18 years of age or older at time of diagnosis of breast cancer (ICD code 174.X) or ductal carcinoma in situ (ICD code 233.0) and a subsequent diagnosis of pregnancy (ICD-9 code V22.2 or alternative pregnancy code) within 5 years of breast cancer diagnosis that were seen at Northwestern between 2000 and 2010. Review of the patients’ electronic medical record was conducted to identify patients that met the inclusion criteria and obtain data on included patients regarding diagnosis, tumor characteristics and staging, treatment history, pregnancy history and outcomes. Disease recurrence included local and metastatic disease.
For the statistical analysis, categorical variables were summarized using frequencies and percentages, and were compared between groups using Fisher’s exact test. Continuous variables were summarized using means, standard deviations, medians and ranges, and were compared between groups using the Wilcoxon rank sum test. Disease free survival (DFS) was summarized using Kaplan Meier curves and compared between groups using the log rank test.
Results
Thirty-two patients were included in the pregnancy cohort and twenty-nine patients in the control (no pregnancy) cohort. There was no statistically significant difference in age, diagnosis, stage, tumor grade or HER2 status between the two groups. More tumors in the control (no pregnancy) cohort were progesterone receptor (PR) positive, 27 (93%) compared to 21(70%) in the pregnancy cohort (P= 0.042). Patient and tumor characteristics are summarized in Table 1.
Table 1.
Patient and tumor characteristics
| Control (not pregnant) (n=29) |
Pregnant (n=32) |
P value | ||
|---|---|---|---|---|
| Diagnosis | DCIS | 7 (24%) | 8 (25%) | 0.99 |
| Invasive Breast Cancer |
22(76%) | 24 (75%) | ||
| Stage | 0 | 7 (24%) | 8 (26%) | 0.84 |
| IA | 11 (38%) | 9 (29%) | ||
| IB | 0 (0%) | 1 (3%) | ||
| IIA | 5 (17%) | 5 (16%) | ||
| IIB | 2 (7%) | 5 (16%) | ||
| IIIA | 4 (14%) | 3 (10%) | ||
| Grade | 1 | 5 (18%) | 6 (21%) | 0.74 |
| 2 | 12 (43%) | 9 (31%) | ||
| 3 | 11 (39%) | 14 (48%) | ||
| PR status | Positive | 27 (93%) | 21 (70%) | 0.042 |
| HER2 status | Positive | 5 (22%) | 4 (14%) | 0.71 |
|
Age (years) at diagnosis |
Mean (range) | 36.1 (26–46) | 34.2 (28–46) | 0.12 |
| BRCA status* | Mutated | 1/17 (6%) | 4/16 (25%) | 0.17 |
Unavailable on 28 patients
Surgical interventions were similar between the cohorts with a trend towards contralateral prophylactic mastectomy in the control (no pregnancy) cohort, but this was not statistically significant. Adjuvant therapy was also similar between the two groups including those that received adjuvant endocrine therapy and radiation therapy. Recurrence Score as analyzed by 21-gene RT-PCR assay and chemotherapy details were not available for most patients and therefore not included in this review. Of the 32 women included in the pregnancy cohort, 19 (63%) received endocrine therapy. In the control (no pregnancy) cohort, 23 of the 28 (82%) women received endocrine therapy (P=0.25). Women were considered to have received endocrine therapy (tamoxifen) even if initiation of treatment was delayed or treatment was interrupted or discontinued due to pregnancy. Treatment interventions are summarized in Table 2. The mean (range) length of endocrine therapy was significantly longer in the control (no pregnancy) cohort, 42.3 (0–120) months, compared to the pregnancy cohort with a mean of 20.9 (0–72) months (P=0.008).
Table 2.
Treatment Interventions
| Control (not pregnant) (n=29) |
Pregnant (n=32) |
P value | ||
|---|---|---|---|---|
|
Surgical management |
Lumpectomy | 16 (55%) | 20 (63%) | 0.61 |
| Mastectomy | 13 (45%) | 12 (38%) | ||
|
Prophylactic contralateral mastectomy |
Yes | 10 (34%) | 4 (13%) | 0.066 |
|
Radiation therapy |
Yes | 17 (59%) | 19 (61%) | 0.99 |
|
Endocrine therapy* |
Treated with endocrine therapy |
23 (82%) | 19 (63%) | 0.25 |
|
Length of Endocrine therapy |
Months Mean(sd) |
42.3 (30.2) | 20.9 (24.0) | 0.008 |
|
Fertility Preservation |
Yes | 2 (7%) | 3 (10%) | 0.99 |
All patients were offered endocrine therapy
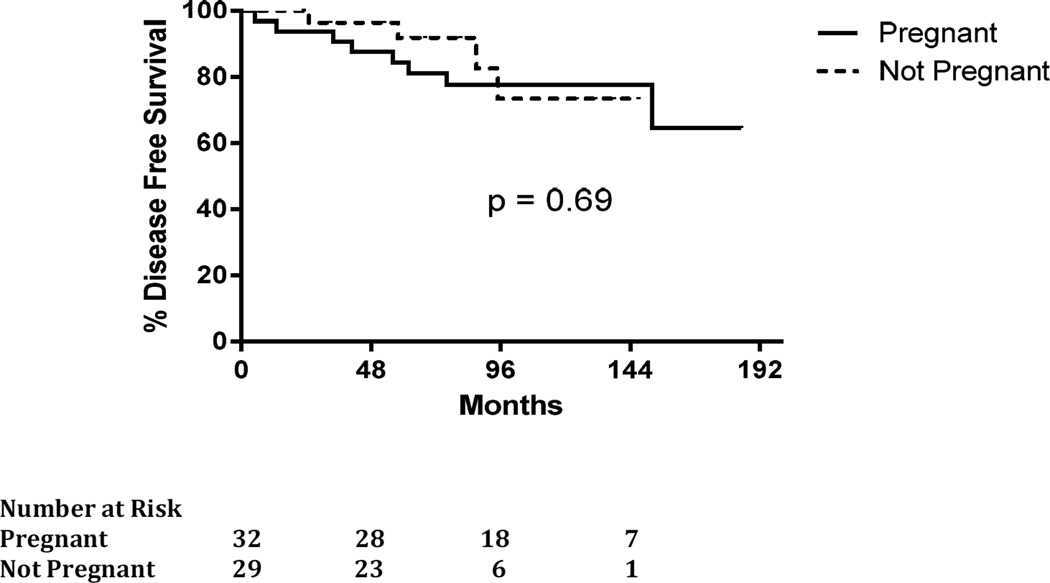
The length of follow up was shorter in the control (no pregnancy) cohort with a mean (range) follow up of 78 (23–168) months compared to 110 (57–185) months in the pregnancy cohort (P=0.005). There was no statistically significant difference in breast cancer recurrence between the women who became pregnant within five years of diagnosis of breast cancer and those that did not. Four women (14%) in the control (no pregnancy) cohort had a breast cancer recurrence compared to eight women (26%) in the pregnancy cohort, (P=0.34). In the pregnancy cohort, nineteen of thirty-two women took adjuvant tamoxifen (63%) – seven taking adjuvant tamoxifen prior to subsequent pregnancy and did not resume after pregnancy, six women delayed tamoxifen until after subsequent pregnancy and six women took tamoxifen for a period of time, held for pregnancy and then resumed to complete adjuvant tamoxifen after pregnancy and breast feeding. In the pregnancy cohort, there were eight breast cancer recurrences, three of which occurred after five years from diagnosis and one of which it is not clear if she had a recurrence or a new primary triple negative tumor. Half of the women with subsequent pregnancy and recurrence did not take adjuvant tamoxifen, one took tamoxifen for 6 months and was diagnosed with recurrence during her subsequent pregnancy and the remaining three women with recurrence took tamoxifen for 18, 24 and 27 months respectively prior to subsequent pregnancy. For women who did not become pregnant within five years of their breast cancer diagnosis, the five-year DFS was 92% (95% CI 81% to 100%). For women who did achieve a pregnancy within five years of their diagnosis of breast cancer, the five-year DFS was 84% (95% CI 72% to 97%). No statistically significant difference was found in the five-year DFS for women who became pregnant within five years of a diagnosis of hormone positive breast cancer compared to women who did not become pregnant within five years after their diagnosis (P=0.69) (Figure 1).
Figure 1.
Disease free survival from diagnosis of breast cancer.
Discussion
There have been several retrospective analyses and meta-analyses aiming to address the question of cancer outcomes after breast cancer and subsequent pregnancy which have demonstrated no adverse effect on overall survival (OS) however much of this data is from the pre-tamoxifen era.4–7 The International Breast Cancer Study Group (IBCSG) conducted a retrospective study looking at patients enrolled in their prior IBCSG trials V, VI and VIII who subsequently became pregnant after diagnosis and compared them to women in the database who did not become pregnant matching for nodal status, tumor size, age and year of diagnosis.3 The women who subsequently became pregnant did not have a worse five- or ten-year overall survival compared to women who did not become pregnant. In a cohort study using population-based cancer registries linked to birth certificate data in three major United States cities, women with a diagnosis of breast cancer and subsequent documented birth were compared to age-, stage- and race-matched women and mortality was assessed.8 There was no increased risk of mortality in this analysis and women who had a birth occurring 10 months or more after their diagnosis of breast cancer had a decreased risk of mortality (RR = 0.54). This may in part be associated with a healthy mother effect, which can be attributed in many studies concerning pregnancy. A subset analysis did identify that women with features associated with high-risk breast cancer, such as larger tumors, lymph node positive or requiring chemotherapy, did have higher risk of mortality. Overall, there was no increased risk of mortality with subsequent pregnancy. Blakely et al conducted a retrospective analysis of women diagnosed with invasive breast cancer at age 35 years old or younger at their Cancer Center in Houston, Texas and the effect of subsequent pregnancy on outcomes.5 Estrogen receptor data was not available on a third of patients, but of the remaining, only 41% had estrogen-receptor positive disease. In this study, the women who went on to have a subsequent pregnancy after their breast cancer diagnosis did not demonstrate an increased risk of breast cancer recurrence or worse overall survival. While there is retrospective data demonstrating no increased risk of recurrence or mortality with pregnancy after breast cancer, these analyses did not specifically look at a hormone receptor-positive cohort or use a control cohort of women who received, or were at least offered, adjuvant tamoxifen therapy which is now the standard of care and associated with a 30–50% decrease in breast cancer recurrence.9,10 We acknowledge that pre-menopausal women diagnosed with breast cancer are more likely to present with hormone receptor-negative disease compared to older women; however two-thirds of young pre-menopausal women will still have estrogen receptor-positive tumors11 and should be offered tamoxifen. It is understandably difficult for physicians to counsel their patients on the risk of holding tamoxifen for childbearing, as tamoxifen is contraindicated in pregnancy, with limited data on outcomes of breast cancer after subsequent pregnancy in the tamoxifen era. As part of a multicenter retrospective study, Azim et al did assess the influence of hormone receptor status on breast cancer outcomes in women with subsequent pregnancy after diagnosis of breast cancer compared to women who did not become pregnant and found no difference in DFS based on tumor hormone status.12 In this study, only 26–27% of patients were treated with anti-hormone therapy at some point during their course. The low use of adjuvant tamoxifen is largely due to timing of data collected prior to tamoxifen becoming the standard of care in management of early stage, hormone receptor-positive breast cancer.
Also causing an intellectual dilemma for physicians counseling their patients is recent investigations supporting strategies to further decrease endogenous estrogen exposure after diagnosis of breast cancer, either by extension of adjuvant anti-hormone therapy or ovarian suppression or ablation in young women. Both the ATLAS and the aTTom trials demonstrated potential benefit in recurrence and mortality with continuing tamoxifen for 10 years in patients with estrogen receptor-positive breast cancer.13,14 The SOFT trial data showed that while there was no benefit to ovarian suppression or ablation in the overall study population of women with early stage, hormone receptor-positive breast cancer treated with tamoxifen, there was a benefit seen in women under the age of 35 who remained premenopausal after chemotherapy.15 The TEXT data further supported ovarian suppression by demonstrating a DFS benefit in women treated with adjuvant exemestane plus ovarian suppression versus tamoxifen plus ovarian suppression.16
The concept of extending the time of anti-estrogen exposure or inducing a postmenopausal state contradicts the concept of safety of pregnancy (and its associated hormonal milieu) after diagnosis of breast cancer although little is understood about the effects of pregnancy on the microenvironment in the breast and whether there may be a protective effect of pregnancy. Some have hypothesized that the steep drop in estrogen level after pregnancy can cause apoptosis in breast cells while others have demonstrated a reduction in estrogen receptor expression in pregnant women that may protect against breast cancer.17,18
Our retrospective investigation did not demonstrate a difference in breast cancer recurrence in women who became pregnant within five years of their diagnosis of early stage breast cancer compared to women who did not become pregnant within five years of their diagnosis. Many women had interrupted their adjuvant endocrine therapy to become pregnant and breast feed after pregnancy and later returned to complete the standard five years of therapy. Overall however, women in the pregnancy cohort had a shorter length of endocrine therapy than women in the control (no pregnancy) cohort despite the control cohort having a shorter length of follow up. We recognize the limitations in the retrospective design of this study including selection and information bias. Our sample size was small and details on the influence of parity and treatment including length of endocrine therapy would be better addressed in prospective trials or registries with larger sample size and longer follow up. There may also be sampling bias, in that healthy women may be more likely to achieve pregnancy and this could potentially influence their breast cancer outcome.
The issue of pregnancy after a diagnosis of breast cancer is an important factor in cancer survivorship and more information is needed to counsel our patients. Pagani et al recently published data from a survey of young patients with breast cancer regarding fertility concerns and interest in a future study of endocrine therapy interruption to allow pregnancy in which many women were interested.19 Based on this information, the Alliance A221405 POSITIVE trial was designed and is a prospective phase II trial investigating breast cancer outcomes with endocrine therapy interruption after breast cancer diagnosis to allow pregnancy.
Conclusion
In conclusion, this study did not demonstrate a difference in breast cancer recurrence in pre-menopausal women with early stage estrogen receptor positive breast cancer that became pregnant within five years of their breast cancer diagnosis compared to women who did not become pregnant during that time. The study was designed using a time period that would only include women who were diagnosed during the time when adjuvant endocrine therapy was the standard of care at our institution. All women included were offered adjuvant endocrine therapy, which makes this study unique from previous retrospective analyses. While our findings are consistent with other reports which have failed to identify a difference in outcomes based on subsequent pregnancy, the sample sizes are small and caution should be exercised in interpreting these data. Further prospective investigation will be beneficial to both patients and physicians in discussions of pregnancy as a key survivorship issue. We believe young women desiring pregnancy after a breast cancer diagnosis should be well informed of the available data and the continued investigation into understanding the influence of pregnancy on breast cancer recurrence risk.
Clinical Practice Points.
Breast cancer is the most commonly diagnosed malignancy in pre-menopausal women over the age of 30 and with a trend in women delaying pregnancy and childbearing until later in life, future pregnancy is of increasing concern. There have been several retrospective analyses and meta-analyses aiming to address the question of cancer outcomes after breast cancer and subsequent pregnancy which have demonstrated no adverse effect on overall survival. However, most of the available data is collected from dates prior to adjuvant tamoxifen being the standard of care for hormone positive breast cancer and significantly improving outcomes. Tamoxifen is contraindicated during pregnancy, so women would need to discontinue tamoxifen to achieve pregnancy and for breast feeding. To our knowledge, there are no studies specifically addressing outcomes in women with estrogen receptor-positive breast cancer. This study did not demonstrate a worse 5-year disease free survival in women who became pregnant within 5 years of their diagnosis of hormone-receptor positive breast cancer despite having a shorter mean length of endocrine therapy. Based on published data from a survey by Pagani et al, young patients with breast cancer are interested in a future study of endocrine therapy interruption to allow pregnancy and the ongoing Alliance A221405 POSITIVE trial will address this question in a prospective phase II study investigating breast cancer outcomes with endocrine therapy interruption after breast cancer diagnosis to allow pregnancy. In the meantime, our results will help inform patients and physicians as they make decisions on future fertility and pregnancy.
Acknowledgments
Funding: This work was supported, in part, by the National Institutes of Health’s National Center for Advancing Translational Sciences (https://ncats.nih.gov/), [Grant Number UL1TR001422]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No conflicts of interest to report.
References
- 1.Cancer Statistics Review, 1975 – 2012 [Google Scholar]
- 2.Matthews TJ, Hamilton BE. Delayed childbearing: more women are having their first child later in life. NCHS Data Brief. 2009;(21):1–8. [PubMed] [Google Scholar]
- 3.Gelber S, Coates AS, Goldhirsch A, et al. Effect of pregnancy on overall survival after the diagnosis of early-stage breast cancer. J Clin Oncol. 2001;19(6):1671–1675. doi: 10.1200/JCO.2001.19.6.1671. [DOI] [PubMed] [Google Scholar]
- 4.Azim HA, Jr, Santoro L, Pavlidis N, et al. Safety of pregnancy following breast cancer diagnosis: a meta-analysis of 14 studies. Eur J Cancer. 2011;47(1):74–83. doi: 10.1016/j.ejca.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 5.Blakely LJ, Buzdar AU, Lozada JA, et al. Effects of pregnancy after treatment for breast carcinoma on survival and risk of recurrence. Cancer. 2004;100(3):465–469. doi: 10.1002/cncr.11929. [DOI] [PubMed] [Google Scholar]
- 6.Ives A, Saunders C, Bulsara M, Semmens J. Pregnancy after breast cancer: population based study. BMJ. 2007;334(7586):194. doi: 10.1136/bmj.39035.667176.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sankila R, Heinavaara S, Hakulinen T. Survival of breast cancer patients after subsequent term pregnancy: "healthy mother effect". Am J Obstet Gynecol. 1994;170(3):818–823. doi: 10.1016/s0002-9378(94)70290-x. [DOI] [PubMed] [Google Scholar]
- 8.Mueller BA, Simon MS, Deapen D, Kamineni A, Malone KE, Daling JR. Childbearing and survival after breast carcinoma in young women. Cancer. 2003;98(6):1131–1140. doi: 10.1002/cncr.11634. [DOI] [PubMed] [Google Scholar]
- 9.Allred DC, Anderson SJ, Paik S, et al. Adjuvant tamoxifen reduces subsequent breast cancer in women with estrogen receptor-positive ductal carcinoma in situ: a study based on NSABP protocol B-24. J Clin Oncol. 2012;30(12):1268–1273. doi: 10.1200/JCO.2010.34.0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Early Breast Cancer Trialists' Collaborative G. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 11.Fredholm H, Eaker S, Frisell J, Holmberg L, Fredriksson I, Lindman H. Breast cancer in young women: poor survival despite intensive treatment. PLoS One. 2009;4(11):e7695. doi: 10.1371/journal.pone.0007695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Azim HA, Jr, Kroman N, Paesmans M, et al. Prognostic impact of pregnancy after breast cancer according to estrogen receptor status: a multicenter retrospective study. J Clin Oncol. 2013;31(1):73–79. doi: 10.1200/JCO.2012.44.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davies C, Pan H, Godwin J, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381(9869):805–816. doi: 10.1016/S0140-6736(12)61963-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richard Gray ea. aTTom: Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years in 6,953 women with early breast cancer. Journal of Clinical Oncology. 2013;31(18 suppl) [Google Scholar]
- 15.Francis PA, Regan MM, Fleming GF, et al. Adjuvant ovarian suppression in premenopausal breast cancer. N Engl J Med. 2015;372(5):436–446. doi: 10.1056/NEJMoa1412379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pagani O, Regan MM, Walley BA, et al. Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N Engl J Med. 2014;371(2):107–118. doi: 10.1056/NEJMoa1404037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asztalos S, Gann PH, Hayes MK, et al. Gene expression patterns in the human breast after pregnancy. Cancer Prev Res (Phila) 2010;3(3):301–311. doi: 10.1158/1940-6207.CAPR-09-0069. [DOI] [PubMed] [Google Scholar]
- 18.Medina D. Mammary developmental fate and breast cancer risk. Endocr Relat Cancer. 2005;12(3):483–495. doi: 10.1677/erc.1.00804. [DOI] [PubMed] [Google Scholar]
- 19.Pagani O, Ruggeri M, Manunta S, et al. Pregnancy after breast cancer: Are young patients willing to participate in clinical studies? Breast. 2015;24(3):201–207. doi: 10.1016/j.breast.2015.01.005. [DOI] [PubMed] [Google Scholar]