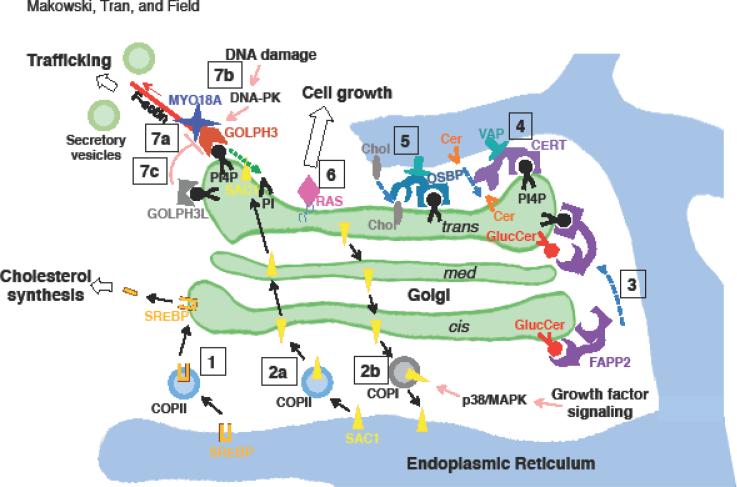
Figure.
Summary of some of the regulatory events that occur at the Golgi, as described in the main text.
[1] When cholesterol levels are low, SREBP is transported by COPII vesicles from the ER to the Golgi. This allows SREBP to be cleaved by Golgi-specific proteases, releasing an N-terminal fragment that enters the nucleus to activate the transcription of genes involved in cholesterol uptake and synthesis.
[2a] In the absence of growth factors, oligomerized SAC1 is transported by COPII vesicles and 14-3-3 from the ER to the Golgi, where it dephosphorylates PtdIns(4)P (PI4P) to produce PtdIns (PI), resulting in reduced secretory trafficking.
[2b] Growth factor signaling activates the p38/MAPK pathway, which stimulates dissociation of SAC1 oligomers and COPI-mediated trafficking of SAC1 from the Golgi to the ER. This increases PtdIns(4)P levels at the Golgi, which drives increased anterograde trafficking.
[3] FAPP2, which localizes to the trans-Golgi through binding to both PtdIns(4)P and ARF, transports glucosylceramide (GlucCer) from the cis- to trans-Golgi to facilitate globotriaosylceramide production.
[4] CERT binds to PtdIns(4)P at the Golgi and VAP at the ER, transporting ceramide (Cer) from the ER to the trans-Golgi at ER-Golgi contact sites.
[5] OSBP binding to PtdIns(4)P at the Golgi and VAP at the ER allows transport of cholesterol (Chol) from the ER to the trans-Golgi at ER-Golgi contact sites.
[6] While RAS signaling is typically thought to occur at the PM, a growing body of literature reports RAS localization to the Golgi, where it may trigger signaling that promotes cell growth.
[7a] The GOLPH3 complex, involving PtdIns(4)P/GOLPH3/MYO18A/F-actin, links the Golgi to the actin cytoskeleton and applies a tensile force to the Golgi membrane which aids in vesicle budding for forward trafficking to the PM.
[7b] DNA damage triggers DNA-PK activation, which phosphorylates GOLPH3 at Thr 143 and enhances its interaction with MYO18A, resulting in Golgi vesiculation and fragmentation. The resulting change in trafficking is required for normal cell survival following DNA damage.
[7c] GOLPH3L asserts a dominant-negative effect on the GOLPH3 complex. GOLPH3L binds to PtdIns(4)P but is unable to bind to MYO18A.