Abstract
Objectives
An important clinical application of transient-evoked otoacoustic emissions (TEOAEs) is to evaluate cochlear outer hair cell function for the purpose of detecting sensorineural hearing loss (SNHL). Double-evoked TEOAEs were measured using a chirp stimulus, in which the stimuli had an extended frequency range compared to clinical tests. The present study compared TEOAEs recorded using an unweighted stimulus presented either at ambient pressure or tympanometric peak pressure (TPP) in the ear canal, and TEOAEs recorded using a power-weighted stimulus at ambient pressure. The unweighted stimulus had approximately constant incident pressure magnitude across frequency, and the power-weighted stimulus had approximately constant absorbed sound power across frequency. The objective of this study was to compare TEOAEs from 0.79 to 8 kHz using these three stimulus conditions in adults to assess test performance in classifying ears as either having normal hearing or SNHL.
Design
Measurements were completed on 87 adult participants. Eligible participants had either normal hearing (N=40; M|F=16|24; Mean Age=30 y) or SNHL (N=47; M|F=20|27; Mean Age=58 y), and normal middle-ear function as defined by standard clinical criteria for 226-Hz tympanometry. Clinical audiometry, immittance, and an experimental wideband test battery, which included reflectance and TEOAE tests presented for one-minute durations, were completed for each ear on all participants. All tests were then repeated one to two months later. TEOAEs were measured by presenting the stimulus in the three stimulus conditions. TEOAE data were analyzed in each hearing group in terms of the half-octave averaged signal-to-noise ratio (SNR) and the coherence synchrony measure (CSM) at frequencies between 1 and 8 kHz. The test-retest reliability of these measures was calculated. The area under the receiver operating characteristic curve (AUC) was measured at audiometric frequencies between 1 and 8 kHz to determine TEOAE test performance in distinguishing SNHL from normal hearing.
Results
Mean TEOAE SNR was ≥8.7 dB for normal-hearing ears and ≤6 dB for SNHL ears for all three stimulus conditions across all frequencies. Mean test-retest reliability of TEOAE SNR was ≤4.3 dB for both hearing groups across all frequencies, although it was generally less (≤3.5 dB) for lower frequencies (1 to 4 kHz). AUCs were between 0.85 and 0.94 for all three TEOAE conditions at all frequencies, except for the ambient TEOAE condition at 2 kHz (0.82) and for all TEOAE conditions at 5.7 kHz with AUCs between 0.78 and 0.81. Power-weighted TEOAE AUCs were significantly higher (p<0.05) than ambient TEOAE AUCs at 2 and 2.8 kHz, as was the TPP TEOAE AUC at 2.8 kHz when using CSM as the classifier variable.
Conclusions
TEOAEs evaluated in an ambient condition, at TPP and in a power-weighted stimulus condition had good test performance in identifying ears with SNHL based on SNR and CSM in the frequency range from 1 to 8 kHz, and showed good test-retest reliability. Power-weighted TEOAEs showed the best test performance at 2 and 2.8 kHz. These findings are encouraging as a potential objective clinical tool to identify patients with cochlear hearing loss.
Introduction
When middle-ear function is normal, reduced levels of evoked otoacoustic emissions (OAEs) are an indication that the ear may have a sensory hearing loss (e.g., Gorga et al. 1997; Keefe et al. 2011). Transient-evoked OAEs (TEOAEs) elicited using a double-evoked method (Keefe 1998) and a stimulus bandwidth from 0.71 to 8 kHz may provide more information about cochlear integrity than traditional TEOAE tests since they extend to higher frequencies (Keefe et al. 2011). The procedure uses a nearly constant level of incident pressure as a function of frequency to calibrate for standing-wave effects that occur in the ear canal (Goodman et al. 2009). Previous studies have evaluated TEOAEs elicited with a chirp stimulus (e.g., Neumann et al. 1994; Bennett & Ozdamar 2010b; Jedrzejczak et al. 2013; Kalluri & Shera 2013; Marshall et al. 2014), although to our knowledge no study to date has used TEOAEs elicited with a chirp to classify ears as having normal hearing or a sensorineural hearing loss.
The use of a chirp stimulus to evoke the TEOAE may be advantageous in comparison with the more commonly used click stimulus. Primarily, the need exists for TEOAE measurements to characterize the cochlear mechanics above the clinical TEOAE test bandwidth that traditionally extends to 4–5 kHz. The transient stimuli used to measure the TEOAE should produce minimal system distortion. In that respect, the chirp is superior to the click because, for a given total energy in the transient, it spreads the energy out in time which produces smaller peak levels. The present methodology is distinct from other OAE studies in which the stimuli were characterized as chirps or swept tones (e.g., Bennett & Ozdamar 2010a; Kalluri & Shera 2013), in that its chirp sweep rate of 174.6 Hz/ms is substantially faster using procedures described by Keefe et al. (2016). While that study reported results only in ears with normal hearing, the present study reports chirp TEOAE results both in ears with normal hearing and ears with sensorineural hearing loss (SNHL).
Since middle-ear function impacts the measurement of OAEs (Trine et al. 1993; Keefe 2007; Sun & Shaver 2009; Sun 2012), it may prove useful clinically to control for variables such as middle-ear pressure or differences in middle-ear function across multiple test visits. A sound presented in the ear canal travels as a pressure wave in the forward direction towards the tympanic membrane (TM), and is partially reflected at the TM and partially absorbed by the middle ear. The latter absorbed energy is conducted via the ossicular chain to the oval window of the cochlea. Part of this energy is absorbed internally within the middle ear, and the remainder forms a forward-transmitted traveling wave within the cochlea. The OAE generated within the cochlea by the forward traveling wave is reverse-transmitted back through the middle ear and ear canal to the probe microphone. Multiple reflections occur within the ear canal as sound waves, and may also occur within the inner ear as cochlear traveling waves (Shera & Cooper 2013).
There can be considerable reductions to both the levels of the stimulus and response in cases of naturally occurring middle-ear dysfunction (Owens et al. 1993), as well as when the air pressure within the sealed ear canal is systematically varied during the test (Osterhammel et al. 1993; Plinkert et al. 1994; Marshall et al. 1997; Konradsson et al. 1999). Such variations in ear-canal air pressure are a model to study the effects of air pressure variations in the middle-ear cavity pressure, as the net force on the TM is based on the difference in the ear-canal and middle-ear cavity pressures acting over the surfaces of the TM. The tympanometric peak pressure (TPP) for a 226-Hz tympanogram is the ear-canal pressure at which the maximum admittance magnitude is achieved. In the normal ear with no under- or over-pressure in the middle-ear cavity, the TPP occurs close to the ambient pressure. Fluctuation in middle-ear pressure away from TPP results in reductions in admittance magnitude, which may reduce OAE levels by decreasing both the level of the forward-directed pressure wave of the OAE-evoking stimulus and the level of the reverse-directed OAE measured by the ear-canal microphone (Naeve et al. 1992; Konradsson et al. 1999; Ellison & Keefe 2005; Prieve et al. 2008; Sun & Shaver 2009; Sun 2012). Thus, controlling for middle-ear pressure by adjusting ear-canal pressure to achieve TPP for the measurement of OAEs may be useful clinically to maximize the evoked OAE signal level.
In addition to adjusting the middle-ear pressure to achieve maximal OAE recordings, adjusting the OAE stimulus level in specific frequency regions to compensate for changes in middle-ear function may be useful. The stimulus amplitude evoking the OAE, as well as the amplitude and phase of the reverse-transmitted OAE, vary with frequency due to individual variability in ear-canal standing waves and middle-ear function (Lonsbury-Martin et al. 1991). Achieving a stimulus adjustment across frequency in an individual ear requires spectral information on ear-canal and middle-ear function.
Wideband (WB) acoustic immittance (WAI) tests possess the ability to measure middle-ear function over the traditional audiometric range (0.2 to 8 kHz) compared to traditional single-frequency middle-ear tests (Feeney et al. 2013). To date, the WAI test that has received the most attention is WB pressure reflectance, which in the frequency domain is the complex ratio of the reverse pressure wave spectrum in the ear canal to the forward pressure wave spectrum. This pressure reflectance has magnitude and phase such that the energy (or power) reflectance is the squared magnitude of the pressure reflectance, and absorbance is one minus this squared magnitude. WB absorbance ranges from zero where no acoustic power is absorbed to one where all acoustic power is absorbed. WB absorbance or power reflectance measurements have been performed at ambient pressure in the ear canal (Keefe & Simmons 2003; Feeney et al. 2014b), and during a WB tympanogram, whereby the absorbance is characterized across both frequency and pressure (Liu et al. 2008; Sanford et al. 2013; Shaver & Sun 2013; Feeney et al. 2014a).
Goodman et al. (2009) and Keefe et al. (2016) described methods for obtaining TEOAE measures at ambient pressure, using a stimulus weighting to produce an approximately constant incident pressure level across frequency. This is called an unweighted stimulus in the present study, and it is presented in both ambient pressure and TPP test conditions. Keefe et al. (2017) generalized this approach to measure TEOAEs using a stimulus that has approximately constant absorbed sound power level across frequency. This is termed the power-weighted stimulus in the present study, for which a WB admittance measurement is required to calculate the absorbed power level based on an initial measurement in the ear canal using the unweighted stimulus. A power-weighted stimulus controls for individual differences in ear-canal standing waves and in the absorbance of the middle ear (Keefe & Schairer 2011). Keefe et al. (2017) reported TEOAE data for a group of adults with normal hearing using a power-weighted stimulus. The purpose of the present study was to apply these methods in a group of adults that included ears with normal hearing and ears with SNHL in order to assess the potential utility to predict the presence of SNHL.
The current study evaluated the use of WB TEOAE responses elicited with a chirp stimulus in three different stimulus conditions: 1) at ambient pressure in the ear canal using an unweighted chirp; 2) at TPP in the ear canal using an unweighted chirp; and 3) at ambient pressure in the ear canal using a power-weighted chirp that was normalized to approximate the same peak-to-peak (pe) SPL as for the unweighted chirp. The power-weighted chirp increased the stimulus level (in relative terms) at frequencies at which the absorbed sound power was low, and decreased the level at frequencies at which the absorbed sound power was high. The accuracy of a TEOAE test to classify ears with SNHL was compared across the three stimulus conditions. All measurements were repeated between one and two months after the initial measurements to assess whether the TEOAE measurements were more reliable in one stimulus condition than the others.
Materials and Methods
I. Participants
To be included in the study, participants had to be adults (18–89 years old) with: 1) normal otoscopy; 2) normal 226-Hz tympanometry (peak-compensated static acoustic admittance from 0.3 to 1.7 mmhos and middle-ear pressure within ±100 daPa); and 3) audiometric air-bone-gaps ≤10 dB HL at octave frequencies from 0.25 to 4 kHz. One hundred and four participants were enrolled in the study. Of these participants, two failed to meet the inclusion criteria, 13 did not have usable data due to equipment issues, and two had an equipment issue or failed the inclusion criterion depending on the study visit. This resulted in 87 participants with usable data.
All procedures were approved by the Institutional Review Board at Oregon Health and Science University and informed consent was obtained from all participants. Clinical audiometric and immittance tests were completed prior to the WB experimental tests at each visit. All testing was completed in a double-walled sound treated booth. Every participant underwent the same testing protocol on both ears during their baseline visit, and at the follow-up visit scheduled approximately one month later. The first ear tested at each visit was determined randomly, and this same ear was tested first during the follow-up visit. Participants who met the inclusion criteria were subsequently tested with the WB experimental system.
II. Clinical Tests and Classification of Hearing Status
A Grason-Stadler GSI-61 audiometer, with ER-3A insert earphones and a B-71 bone-conduction oscillator calibrated to ANSI standards (S3.6, 2010), was used to perform pure-tone audiometry. A Grason-Stadler GSI Tympstar tympanometer calibrated to ANSI standards (S3.39, 2012) was used for 226-Hz tympanometry. The following audiologic tests were administered for both ears of each participant: 1) pure-tone air conduction audiometry at frequencies from 0.25 to 8 kHz; 2) pure-tone bone conduction audiometry from 0.25 to 4 kHz; and 3) tympanometry using a 226-Hz probe tone.
The study participants were classified as having either normal hearing or SNHL in each ear at each audiometric test frequency based upon the following criteria for normal hearing: pure-tone air conduction thresholds ≤ 20 dB HL at any test frequency. The participants with normal hearing in both ears at all frequencies (N=40) ranged from 18 to 51 years (mean age=30 y), and consisted of 16 males and 24 females. The participants with SNHL in at least one ear at one frequency (N=47) ranged from 28 to 81 years (mean age=58 y), and consisted of 20 males and 27 females.
For the purpose of the results described herein, the hearing status was classified for each participant’s ear at each test frequency. Specifically, ears were assigned to either the normal-hearing group or SNHL group based upon the pure-tone findings at a given test frequency, and then ears were re-classified in the same manner at all other test frequencies.
III. Experimental Hardware and Calibration Procedures
Experimental tests were completed using an Interacoustics WB tympanometry research system with modified firmware described in Liu et al. (2008) and Keefe et al. (2015). Briefly, a modified Interacoustics AT235 tympanometer was controlled by custom software via a serial port (RS-232) in a 32-bit computer. The computer sound card (CardDeluxe) had two digital-to-analog converters (DACs) to drive a pair of receivers in a Titan probe assembly (Interacoustics). One receiver was used to measure reflectance and admittance, and both receivers were used to produce the TEOAE stimuli. There was also a channel for analog-to-digital conversion to record the microphone output, with a sampling rate of 22.05 kHz. The system probe was calibrated for absorbance and admittance measurements at the probe tip prior to measurements in human participants by measuring the incident pressure spectrum in a long tube. The tube had an inner diameter of 0.8 cm, which led to a similar cross-sectional area of the tube compared to that of an average adult ear canal. The incident pressure of the stimulus and the source reflectance spectra of the probe were calculated using data from long (~292 cm) and short (~8 cm) tubes in terms of a transmission-line model of sound propagation within each tube (Liu et al. 2008).
The air pressure within the ear canal was measured using a pressure sensor within the tympanometer cable that extended to the tympanometer. Using a bi-directional serial port, a pressure controller circuit matched the actual air pressure in the ear canal to the desired air pressure, whether the desired pressure was fixed (e.g., at ambient pressure or TPP) or swept between an initial and final pressure.
IV. WB Reflectance and Admittance Procedures
The WB reflectance and admittance at the probe tip were measured prior to all TEOAE measurements. The ambient-pressure reflectance and admittance test procedures are first described. The probe tip was inserted into the ear canal, and the air pressure was set to ambient pressure by the system pressure pump to adjust for any over-pressure that may have resulted from the insertion of the probe tip. The admittance and the pressure reflectance of the ear were measured at ambient pressure in the ear canal using a time-averaged click stimulus delivered at an approximate SPL of 62 dB. The acoustic conductance, which was used to calculate the power-weighted stimulus in the subsequent TEOAE tests, was calculated as the real part of the acoustic admittance at the probe tip. An example measurement (as absorbance) is illustrated in Figure 1.
Figure 1.
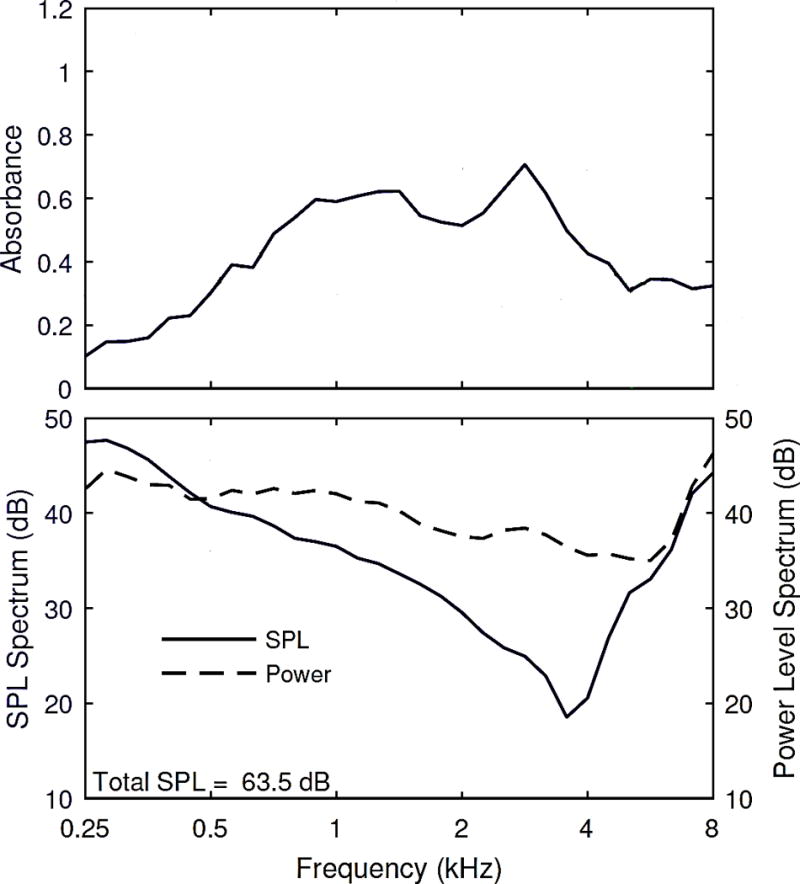
The wideband absorbance pattern (top) as a function of frequency for an adult ear with normal hearing. SPL and power levels (dB) across the spectrum as well as the total SPL are also illustrated (bottom).
The test procedures of the absorbance tympanogram are next described, for which the goal was to measure the TPP of the ear. This TPP was used in subsequent TEOAE tests performed at TPP in the ear canal. The absorbance tympanogram was measured using the same repeated click stimulus as in the ambient-pressure test, but the clicks were presented in the tympanometric test while the air pressure was swept in the ear canal across a range of pressures. Descending and ascending swept tympanograms were each measured with extremal pressures of −200 and +200 daPa. The tympanograms were obtained using a nominal sweep rate of 100 daPa/s for both tympanograms, for which the air pressure was sampled every 25 ms while clicks were repeatedly delivered with an inter-click interval of 46 ms. If the system was unable to pressurize up (or down) to the initial extremal pressure within 5 s, or if the entire pressure sweep was not completed within 12 s, then a message informed the operator that the probe seal had a leak and needed to be refitted prior to repeating the test. The TPP of the ear was calculated as the average TPP for the descending and ascending absorbance tympanograms, in which each was averaged over a low-frequency bandwidth between 0.376 and 2 kHz. This averaging procedure substantially reduced middle-ear hysteresis effects associated with the polarity of the sweep (Liu et al. 2008).
These reflectance and admittance measurement procedures at ambient pressure and as swept tympanograms are described in detail in Keefe et al. (2015). Normative measurements of reflectance and admittance in a group of adult subjects with normal hearing are described in (Feeney et al., 2016).
V. TEOAE Procedures
TEOAE testing was performed in each ear following reflectance and admittance measurements using the same probe. The standard procedure was to use the same probe insertion used in the reflectance and admittance measurements, although this was not always possible if the subject took a break or if the probe was removed for some other reason. The duration of each TEOAE test was approximately one minute.
The first TEOAE test was obtained at ambient pressure in the ear canal using the unweighted chirp spectrum. As described above, the unweighted chirp had an incident pressure spectrum level that was approximately constant across frequency over a measurement and analysis bandwidth from 0.71 to 8 kHz. This incident pressure spectrum was measured by inserting the probe into a reflection-less tube, i.e., one whose length (30.5 m) was sufficiently long that any reflected energy from the far closed end of the tube arrived at a time later than the duration of the transient stimulus. This coiled polyethylene tube had an area similar to that of an average adult ear canal. This approach to calibrating the sound stimulus in terms of incident sound pressure has been used in previous TEOAE studies (Goodman et al. 2009; Keefe et al. 2016).
Approximately 20% of the initially tested control and SNHL ears used a chirp stimulus with energy between 0.71 and 10 kHz, although all results from these ears were analyzed over the bandwidth from 0.71 to 8 kHz. As with the other two chirp types, TEOAEs were analyzed using a double-evoked paradigm (Keefe 1998; Keefe & Ling 1998) with a so-called positive chirp stimulus to signify that its local frequency increased with increasing time during the chirp (Keefe et al. 2016). A measured positive chirp at ambient pressure in the ear canal in a normal adult ear is shown in Figure 2 in the top left panel. This plot shows data from the same participant ear as for the absorbance, and the SPL and absorbed power spectra shown in Figure 1. The sweep rate of the chirp with bandwidth 0.71 to 8 kHz was 174.6 Hz/ms, so that the effective duration of the chirp to sweep from 0.71 to 8 kHz was about 42 ms (the sweep rate of the chirp with bandwidth 0.71 to 10 kHz was similar).
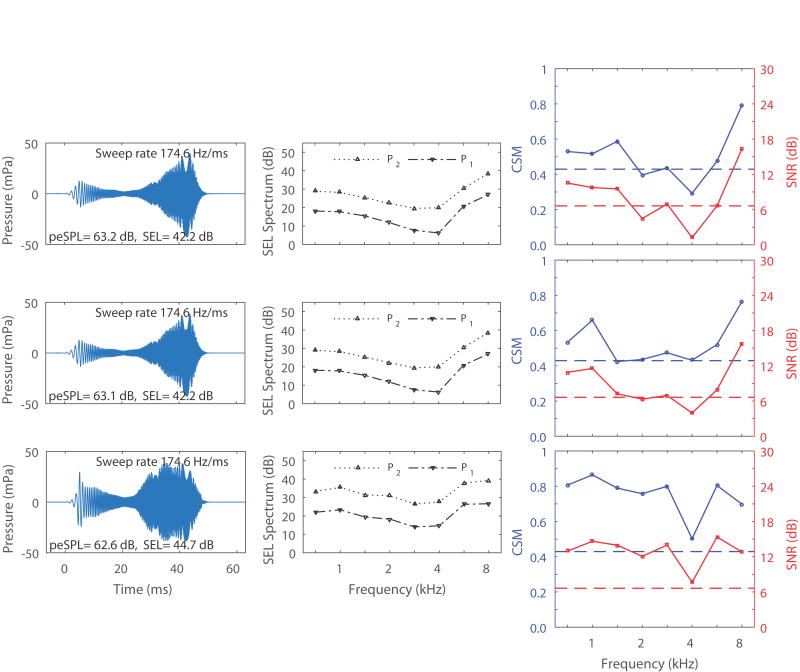
Figure 2.
The left panels are the time waveforms for the unweighted ambient chirp (top), unweighted chirp at TPP (middle), and power-weighted ambient chirp (bottom) in the same adult ear with normal hearing from Fig. 1. TPP was +15 daPa for this ear. The stimulus swept in time from 0.71 to 8 kHz in approximately 42 ms. The center panels are the sound exposure levels (SEL) for stimulus 1 (P1, negative triangle) and stimulus 2 (P2, positive triangle) as a function of frequency for the same three chirp conditions respectively (top, middle, and bottom). The total SEL for each chirp is displayed in the lower-right corner of the left panels. The right panels are the resulting TEOAE SNR (dB) (solid lower line) and CSM (solid upper line) for the same three chirp conditions respectively. The lower and upper dashed lines in each panel represent the critical SNR and CSM respectively, corresponding to a detection criterion at p=0.05.
The chirp was output from DAC1 at the reference level and from DAC2 at a level 12 dB larger than the reference level with the same stimulus polarity. A double-evoked TEOAE waveform was extracted from the sum of the responses to the DAC1 stimulus alone and the DAC2 stimulus alone, minus the response to the simultaneous presentation of the stimuli from DAC1 and DAC2 (Keefe 1998). In this procedure, the three stimuli had the same buffer duration, and the location of each chirp within each buffer was the same in DAC1 and DAC2.
In the simultaneous presentation condition, the chirp stimulus amplitude is approximately equal to the sum of the amplitudes output by DAC1 and DAC2, because the underlying chirp stimulus waveform is the same, aside from a multiplicative gain factor. A level increase of 12 dB for DAC2 corresponds to a multiplicative gain of approximately 4 relative to the DAC1 nominal gain of 1. Their summed amplitude gain is thus approximately 5 relative to the amplitude associated with DAC1, which corresponds to a relative level of about 14 dB for the simultaneous presentation. The stimulus level reported in the present study was based on the peak-to-peak equivalent sound pressure level (peSPL) across all three buffers, so that it was controlled by the peSPL for the simultaneous presentation condition. This is in contrast to the chirp stimulus level measure reported in Keefe et al. (2016) and Keefe et al. (2017), in which the peSPL was based on the chirp sound produced by the receiver driven by DAC1. Thus, the peSPLs in the present study are reported as about 14 dB higher than in those studies. Other than this difference in how the peSPL was reported, the positive chirp amplitude was similar in all these studies.
This double-evoked procedure reduced transducer distortion compared to clinical TEOAE procedures (Keefe & Ling 1998). The rapid TEOAE sweep rate allowed multiple responses to be time- and spectral-averaged, and also facilitated the rejection of individual chirp responses potentially contaminated by artifact as described in (Keefe et al. 2016). Particularly, the data were highpass filtered in real time using a Kaiser window finite impulse response (3 dB and 40 dB attenuation at 0.66 and 0.4 kHz, respectively), and a median absolute deviation (MAD) test was employed to reject response buffer outliers of amplitude, crest factor, and noise energy (Keefe et al. 2016).
The second TEOAE test was obtained in the same manner as the first (see Figure 2, middle left panel), except that it was performed at the ear TPP measured in the low-frequency averaged absorbance tympanogram. During the one-minute test, the actual air pressure in the ear canal was monitored by the pressure controller circuit described above. The desired air pressure was fixed at the ear TPP. TEOAE data were acquired in intervals of 6 s, and analyzed to provide the operator with quasi real-time updates on the signal and noise levels of the stimulus and the TEOAE. The actual air pressure was monitored at each 25 ms within the test, and the test was automatically paused if the desired and actual pressure differed by more than 10 daPa. In such a pause, the operator was alerted to check the quality of the probe fit and restart the test as needed. Intermittently noisy data were rejected based on the MAD test described above. The stored TEOAE data at TPP were otherwise analyzed in the same manner as the TEOAE at ambient pressure, and over the same one-minute duration.
The third power-weighted TEOAE test was obtained at ambient pressure with the chirp stimulus level adjusted to account for differences in the sound power absorbed by the ear across frequency. Inasmuch as the sound power loss at the ear-canal walls is much smaller than the sound power loss at the TM, the sound power absorbed by the ear is dominated by the power absorbed by the middle ear. To produce the power-weighted chirp, the ambient conductance at the probe tip was obtained as the real part of the measured admittance at the probe tip. As explained in Keefe et al. (2017), the chirp stimulus waveform (Figure 2, bottom left panel) was adjusted in amplitude at each time step so that the resulting absorbed sound power spectrum was approximately constant over the frequency range from 0.25 to 8 kHz. This power-weighted chirp stimulus was then scaled by a single gain factor applied to the DAC waveform to generate approximately the same peSPL in the ear as was measured using the unweighted chirp (i.e., the ambient chirp described above). Note that the power-weighted stimulus levels have a flatter morphology (Figure 2, bottom middle panel) because the levels are boosted primarily where they are the lowest in the other two chirp conditions (from ~2.8 to 5.7 kHz).
For each stimulus condition, two measures were used to assess the presence and strength of the TEOAE response. First, the TEOAE signal-to-noise ratio (SNR) was calculated for each half-octave frequency from 0.79 to 8 kHz. The second measure was a coherence synchrony measure (CSM) based on the degree of synchrony of the TEOAE across the multiple buffers holding the TEOAE responses acquired in the one-minute recording (Keefe 2012). CSM varies from one representing perfect synchrony to zero representing no synchrony. CSM is closer to one if the spectral phasor angles calculated from the complex TEOAE spectra in the multiple buffers are highly similar to one another. These measures were calculated in an identical manner to the methodological discussion in Keefe et al. (2016). The TEOAE SNR (dB) and CSM are presented in the right panels of Figure 2 for each stimulus condition. As described in Keefe (2012), a TEOAE is classified as present based on CSM if the measured CSM is the same or greater than the critical CSM corresponding to a detection criterion at p=0.05. A TEOAE is classified as present based on SNR if the measured SNR is the same or greater than the critical SNR (6.6 dB) corresponding to a detection criterion at p=0.05. Note that the TEOAEs would be considered either absent or borderline present at several frequencies in the middle of the spectrum for the unweighted ambient and unweighted TPP chirp conditions (top and middle), while the power-weighted chirp TEOAEs (bottom) would be considered present at all frequencies using either SNR or CSM.
VI. Statistical Analyses
TEOAEs were analyzed in half-octave frequency bands. A linear mixed model approach using repeated measures analysis of variance was used for data analyses for SNR and CSM measures. This statistical approach was employed to estimate the reliability of test-retest data while including data from subjects who may not have completed a repeat visit (McMillan et al. 2013; Reavis et al. 2015). The linear mixed model assumes that the initial and follow-up test measurements (yi1 and yi2) are bivariate Gaussian random variables and share homeostasis, such that there is a common mean (μi), variance ( + ), and covariance ( ) (McMillan 2014). Under the above assumptions, the absolute test-retest differences were calculated using Statistical Analysis Software (SAS). An estimate of is utilized in this case, whereby the absolute test-retest difference (|yi1 – yi2|) possesses a “Half-Gaussian” distribution and the mean is equal to 2 . This statistic is referred to as the “reliability” with smaller values representing better test-retest reliability (McMillan et al. 2013).
Receiver operating characteristic (ROC) curve analyses were used to determine the sensitivity of each TEOAE method and response measure (SNR and CSM) to classify each ear as having normal hearing or SNHL. The area under the ROC curve (AUC) is an indicator of diagnostic performance to identify SNHL at each half-octave frequency, with 0.5 equaling chance performance and 1 equaling perfect performance. Specifically, the AUC reflects the sensitivity and specificity of each TEOAE band across all test criteria. A nonparametric approach was utilized for the statistical comparison of AUCs across test conditions (DeLong et al. 1988).
Results
A total of 156 ears from 87 participants contributed usable data for the findings presented in this study. Table 1 illustrates the distribution of these 156 ears to each hearing category (normal hearing or SNHL) at each audiometric test frequency. Of these, a total of 105 ears from 67 of the participants had two valid test sessions to contribute to repeated measures analyses. To determine if a session was valid, a minimum of two observers independently reviewed the first and last WB absorbance tympanograms for characteristics inconsistent with a biological absorbance pattern such as a steep and narrow notch or absorbance of zero across most of the spectrum, or a change during the session from the first to last tympanograms. These characteristics were associated with limitations in probe performance, and are consistent with probe effects described below in TEOAE measurements. Any remaining invalid sessions were due to clinical tympanometric and/or audiometric findings that fell outside of the inclusion criteria. If only one ear passed the clinical inclusion criteria on the first visit then both ears were still tested at both visits and any valid sessions were included.
TABLE 1.
Numbers of ears with normal hearing or SNHL at audiometric frequencies from 1 to 8 kHz (total of 156 ears).
| # of Ears with Normal Hearing or SNHL
| ||
|---|---|---|
| Frequency (kHz) | Normal Hearing | SNHL |
| 1 | 140 | 16 |
| 2 | 134 | 22 |
| 3 | 122 | 34 |
| 4 | 118 | 38 |
| 6 | 102 | 54 |
| 8 | 101 | 55 |
The criterion for the normal hearing group was a hearing threshold of ≤20 dB HL.
Box and whisker plots are shown in Figure 3 for the pure tone thresholds for the normal-hearing and SNHL groups at audiometric frequencies from 1 to 8 kHz obtained from the first valid test session for each of the 156 ears. For the single visit findings presented here (and in Figures 5 and 8), the first visit was always chosen if two valid test sessions were available for a given ear, whereas the second visit was chosen if it was the only valid test session. The range of the data was smaller for the normal-hearing group at each frequency due to the restricted range of normal hearing thresholds. Mean and median values for the normal-hearing group generally fell between 5–10 dB HL irrespective of test frequency. The SNHL group included ears representing a broad range of hearing thresholds, particularly at 8 kHz where hearing thresholds ranged from 25 to >80 dB HL. Mean and median values for the SNHL group were between about 35–45 dB HL across frequency, with greater hearing loss at higher frequencies.
Figure 3.
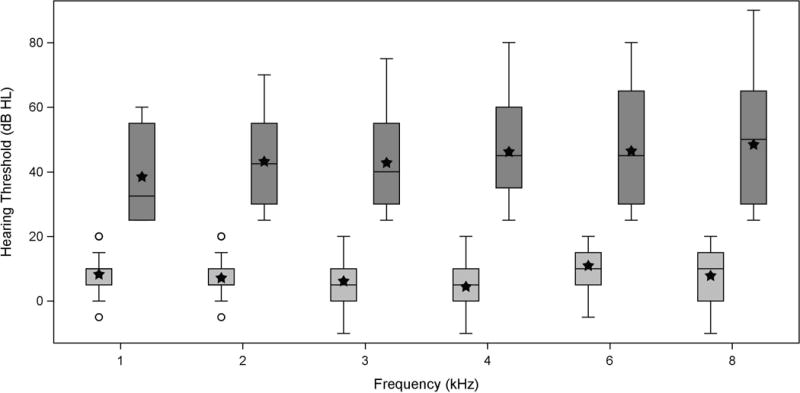
Box and whisker plots for pure-tone threshold data from 1 to 8 kHz in dB HL obtained at the first valid session from the two test sessions for ears with normal hearing (light gray boxes; ≤20 dB HL) and SNHL (dark gray boxes; >20 dB HL). Each shaded box is an interquartile range (IQR) from the 25th to 75th percentiles. The horizontal line within each box is the median, while the filled stars represent the mean for the normal hearing group and SNHL group, respectively. Whiskers denote data within the lesser of ±1.5 times the IQR or the full data range, and any circles (normal hearing) or crosses (SNHL) that are beyond the whiskers represent outliers.
The model-based estimates of mean test-retest differences (i.e., the reliability) for audiometric thresholds are shown in Figure 4 for normal-hearing and SNHL groups, where a smaller number indicates better test-retest reliability. As previously described within the statistical methods, the repeated measures linear mixed model approach used all available data even if there was only one valid test session available for a given ear. The number of ears in each group varies with frequency (see Table 1). Mean pure-tone audiometry reliability was ≤3.6 dB from 1 to 3 kHz for the normal-hearing and SNHL ears. Between 4 and 8 kHz, test-retest differences increased with frequency for the SNHL group, with model estimates of 4.3 dB at 4 kHz and 5.7 dB at 8 kHz. Reliability increased (i.e., was poorer) at frequencies above 4 kHz for both groups. Contrasts between the means for normal-hearing and SNHL ears did not demonstrate a significant difference for test-retest reliability at any of the audiometric frequencies. These test-retest differences were consistent with the accepted clinical standard of ±5 dB.
Figure 4.
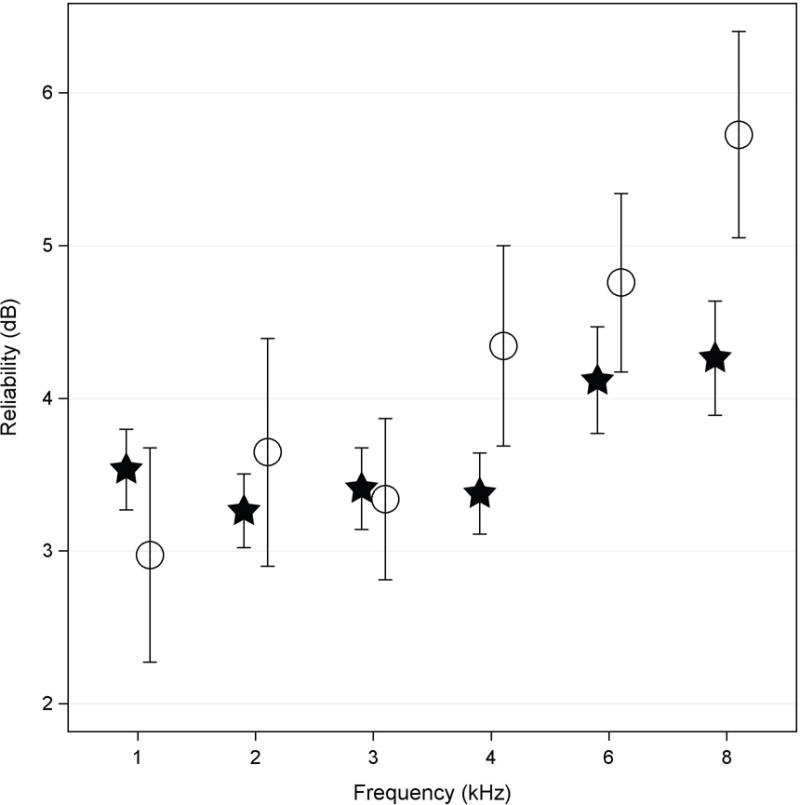
The model-based mean estimate (see Methods for details) of absolute test-retest reliability for pure-tone hearing thresholds from ears with normal hearing (filled stars) and SNHL (open circles). The error bars represent ±1 SE of the mean.
For TEOAEs obtained at the first valid test session, box and whisker plots are shown in Figure 5 for TEOAE SNR (top panels) and CSM (bottom panels) for each TEOAE stimulus type: Ambient (left panels), TPP (middle panels), and Power Weighted (right panels). Within each plot, TEOAEs are shown for the normal-hearing ears and SNHL ears at each test frequency closest to the audiometric frequency (Table 1). Similar to Figure 3, the mean value is added to each box and whisker plot, in which the median is shown as the horizontal line within the box, to better characterize the distribution of the data.
In Figure 5, median and mean TEOAE SNR values for the normal-hearing group in the ambient TEOAE condition (top left panel) were similar at each test frequency. Across frequency the mean TEOAE SNR varied from 8.7 dB at 2 kHz to 13.4 dB at 8 kHz. There was greater variability in SNR within each frequency for normal-hearing ears compared to ears with SNHL. The interquartile range (IQR) for SNR for normal-hearing ears varied from 7 to 12 dB compared to an IQR of as little as 2 to 3 dB for the ears with SNHL depending on the test frequency. The number of outliers for the SNHL ears increased with the test frequency. Despite this, the mean SNR for the SNHL group was 6 dB or less at all frequencies.
Figure 5.
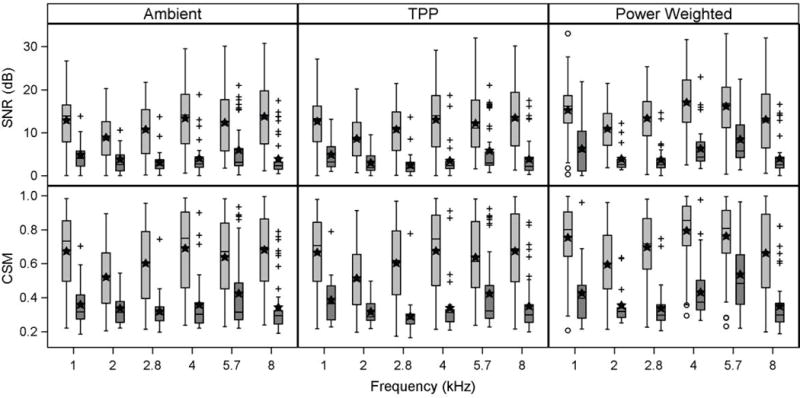
Box and whisker plots for the TEOAE SNR (top panel) and CSM (bottom panel) from data obtained at the first valid session of the two test sessions for ears with normal hearing (light gray boxes) and ears with SNHL (dark gray boxes) at each half-octave frequency for three TEOAE conditions: Ambient pressure (left), TPP (middle) and Power Weighted (right). For each panel the horizontal line within each box is the median, while the filled stars represent the mean for the normal hearing group and SNHL group, respectively. Whiskers denote data within the lesser of ±1.5 times the IQR or the full data range, and any circles (normal hearing) or crosses (SNHL) that are beyond the whiskers represent outliers.
The CSM results in Figure 5 for the ambient TEOAE condition (bottom left panel) demonstrated similar trends as those described for the SNR. CSM median and mean values for the normal-hearing group were greater than 0.5 at all frequencies. There was a greater range of CSM IQR values for the normal-hearing ears than for SNHL ears, as was the case for the SNR IQR. Median and mean CSM were similar to each other at each frequency for the normal-hearing ears whereas mean values were larger than the median in the SNHL ears. CSM median and mean values for the SNHL group were in the range from 0.3 to 0.4. The median and mean CSM for the normal-hearing ears were about two to three times larger than for the SNHL ears across frequencies.
For the TPP TEOAE condition, median and mean for SNR (top middle panel) and CSM (bottom middle panel) were similar across frequencies to the ambient TEOAE condition. For example, the TPP TEOAE mean SNR for the normal-hearing and SNHL groups were all within 0.5 dB compared to the ambient TEOAE condition. In contrast, for the normal-hearing ears, the power-weighted TEOAE condition revealed median and mean SNR values (top right panel) that were 2–4 dB larger than for the other two TEOAE conditions. Additionally, CSM values (bottom right panel) were 0.07–0.12 larger than for the other two TEOAE conditions at each frequency, except at 8 kHz where median and IQR values across TEOAE conditions were nearly identical. IQRs of SNR and CSM for the normal-hearing group in the power-weighted condition were slightly smaller below 8 kHz than for the two other conditions, particularly for CSM. The power-weighted condition also boosted mean and median SNRs by 2–3 dB for the SNHL group relative to the other TEOAE conditions at 1, 4, and 5.7 kHz. The IQRs of SNR at these frequencies were larger as well. For all TEOAE conditions, the SNR and CSM IQRs at 5.7 kHz overlapped (or nearly overlapped) for the normal-hearing and SNHL groups.
The mean TEOAE SNR (top panel) and CSM (bottom panel) for both hearing groups for the three stimulus methods are presented in Figure 6. Data are plotted in this manner for the purpose of comparing the mean SNR and CSM values produced by the three TEOAE stimulus methods for the two hearing types within each test frequency. Contrasts between the mean SNR estimates for the normal hearing group revealed that the power-weighted mean SNR was significantly larger than the ambient and TPP mean SNRs (p<0.0001) from 1 to 5.7 kHz. At 8 kHz the power-weighted mean SNR was significantly smaller than the ambient mean (p<0.01) and the TPP mean (p<0.05). The power-weighted mean SNR was significantly larger than the ambient and TPP means for the SNHL group (p<0.01 to p<0.0001) at nearly all test frequencies. The exceptions were 2 kHz where only the power-weighted mean was larger than the TPP mean (p<0.05), and at 8 kHz where there were no significant differences between the means for any of the three stimulus methods. Contrasts of the mean CSM estimates produced essentially identical findings as those for the mean SNR contrasts. The general trend in the data was that the power-weighted condition produced larger mean SNR and CSM at all frequencies except 8 kHz.
Figure 6.
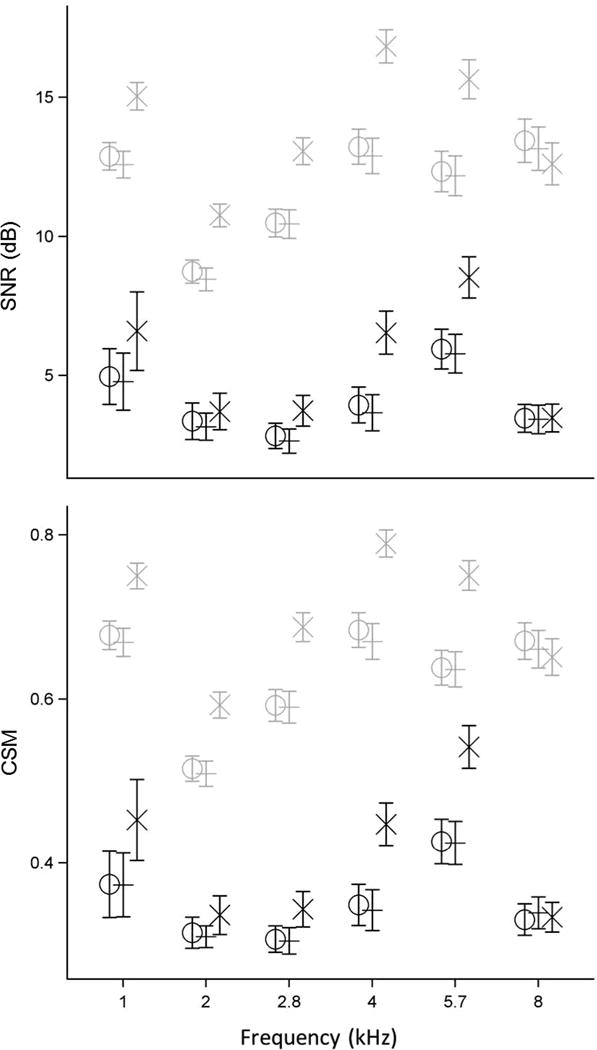
The model-based mean estimate of TEOAE SNR (in dB, top panel) and CSM (bottom panel) at each half-octave frequency for ears with normal hearing (light gray) and SNHL (dark gray). Mean findings for the three TEOAE stimulus methods are plotted together at each frequency and staggered for clarity: Ambient (O), TPP (+), and Power Weighted (X). Error bars in both panels represent ±1 SE from the mean. Some data are slightly displaced horizontally to improve clarity.
This finding is related to the fact that the power-weighting technique is designed to re-arrange the stimulus energy across frequencies, while maintaining a similar peSPL (Keefe et al. (2017)). It should be noted that while the mean peSPL was essentially the same (within 0.2 dB) between the three stimulus methods, the mean SPL of the stimulus was roughly 2 dB greater for the power-weighted method (see Table 2, which also shows the standard error (SE) of the mean). This larger mean SPL was a consequence of the overall re-distribution of stimulus energy in the power-weighted condition relative to the unweighted condition, which was likely responsible for the increased mean SNR and CSM of the TEOAE for the power-weighted condition.
TABLE 2.
The mean (±1 SE) of the SPL and peSPL of the positive chirp for each of the three TEOAE stimulus conditions: Ambient, TPP, and Power Weighted.
| Measurement | Mean SPL (dB) |
Mean peSPL (dB) |
|---|---|---|
| Ambient | 64.3 (±0.1) | 76.4 (±0.1) |
| TPP | 64.7 (±0.1) | 76.4 (±0.1) |
| Power Weighted | 66.8 (±0.1) | 76.6 (±0.1) |
Values represent all valid test sessions for the 156 ears contributing to the data.
The reliability estimates the degree to which the SNR and CSM estimated from TEOAE measurements were repeatable in the same test ear. The model-based estimate of mean test-retest reliability for SNR (top panels) and CSM (bottom panels) at each frequency for each hearing group is shown in Figure 7. Data are separately plotted for each of the three TEOAE conditions: Ambient (left), TPP (middle), and Power Weighted (right). Mean SNR test-retest reliability was ≤4.3 dB for both hearing groups at all test frequencies for all three stimulus methods, although it was generally better (≤3.5 dB) for lower frequencies (1 to 4 kHz). Reliability was generally poorer for the normal-hearing group than for the SNHL group in all three TEOAE conditions. Specifically, mean SNR reliability for the normal-hearing group was poorer than the SNHL group for the ambient TEOAE condition at 2.8 (p<0.01), 4 (p<0.001), and 8 kHz (p<0.0001), for the TPP TEOAE condition at 4 (p<0.0001) and 8 kHz (p<0.0001), and for the power-weighted TEOAE condition at 1 (p<0.05), 2.8 (p<0.0001), 4 (p<0.05), and 8 kHz (p<0.0001). This likely resulted from the fact that the SNRs had a smaller range for SNHL ears compared to normal-hearing ears. Mean SNR reliability also tended to worsen as a function of frequency particularly for the normal hearing group, but also for the SNHL group in the power-weighted condition except for 8 kHz. This trend was similar what was found for mean pure-tone threshold reliability, although in that case the reliability was poorer for the SNHL group compared to the normal-hearing group.
Figure 7.
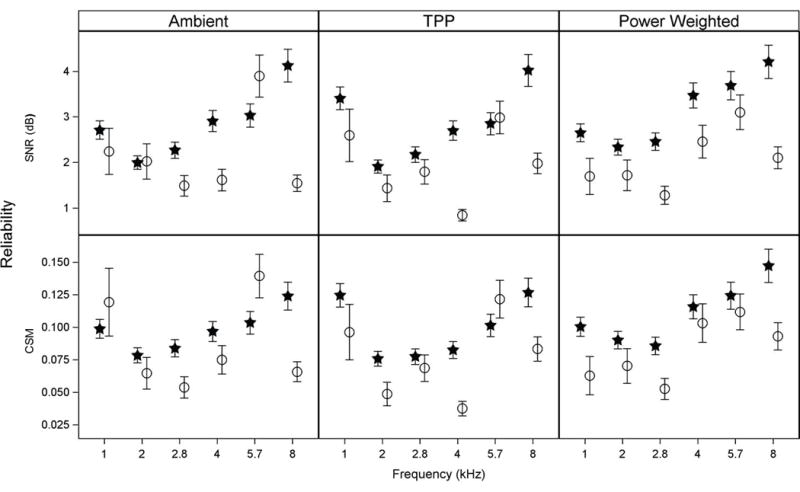
The model-based mean estimate of absolute test-retest reliability for TEOAE SNR (top panel) and CSM (bottom panel) at each half-octave frequency for ears with normal hearing (filled stars) and SNHL (open circles). Data are separately plotted for each of three TEOAE stimulus methods: Ambient (left), TPP (middle), and Power Weighted (right). Error bars represent ±1 SE from the mean.
Mean CSM test-retest reliability, depicted in the bottom panel of Figure 7, demonstrated similar trends to those described for the SNR findings in the top panel. Reliability ranged from 0.035–0.15 for both hearing groups at all test frequencies and for all three stimulus methods. Overall, the CSM reliability worsened as a function of frequency, especially in the normal hearing group. Specifically, reliability for the normal-hearing group was significantly poorer than the SNHL group for the ambient TEOAE condition at 2.8 (p<0.01) and 8 kHz (p<0.0001), for the TPP TEOAE condition at 2 (p<0.05), 4 (p<0.0001) and 8 kHz (p<0.01), and for the power-weighted TEOAE condition at 1 (p<0.05), 2.8 (p<0.01), and 8 kHz (p<0.01).
The ability of TEOAEs to classify ears as having normal hearing or a SNHL was assessed using the AUC. AUC findings are provided in Figure 8 for SNR (left panel) and CSM (right panel) at the TEOAE half-octave frequency closest to the audiometric frequency for all three TEOAE conditions. AUCs across the three stimulus conditions for SNR (left panel) were ≥0.85 at 1, 2.8, 4 and 8 kHz with a high of 0.93 for the power-weighted condition at 2.8 kHz.
Figure 8.
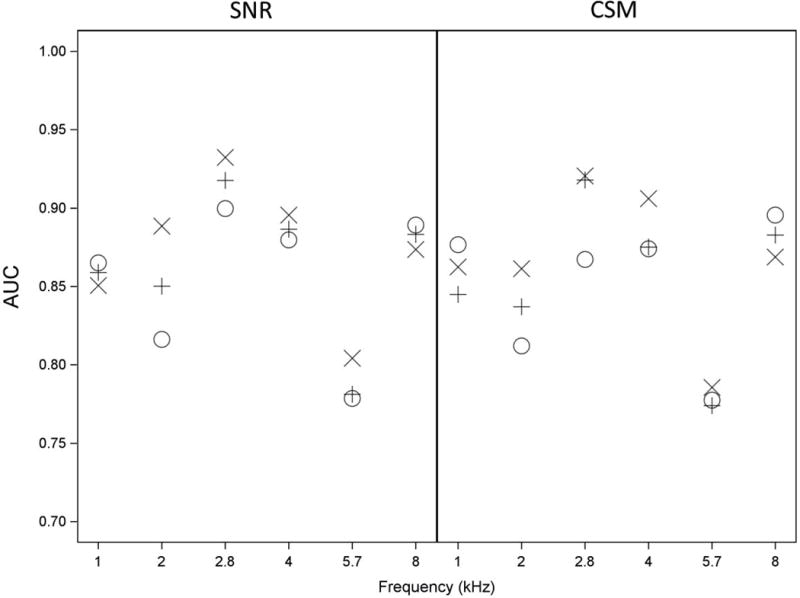
AUCs describe how well ears are classified as having normal hearing or having SNHL at each half-octave frequency based upon TEOAE SNR (left panel) and CSM (right panel) using three TEOAE stimulus methods: Ambient (O), TPP (+), and Power Weighted (X). The number of ears providing data at each frequency is shown in Table 1. Data represented are from the first valid test session from each ear in the dataset.
AUCs were lower at 2 kHz for the ambient TEOAE condition (0.82), and lower still at 5.7 kHz for all three TEOAE conditions (0.77 to 0.81). AUCs for SNR for each TEOAE condition were similar to one another at every frequency except at 2 kHz. At 2 kHz, the AUC for SNR in the power-weighted TEOAE condition (0.89) was significantly higher than the AUC in the ambient TEOAE condition (0.82) (p=0.02). There were no other significant differences between conditions for SNR. In general, CSM produced similar AUC values to SNR for each TEOAE condition at each frequency. It follows that CSM and SNR were equally accurate classifiers of ears into normal hearing and SNHL groups.
AUC values for CSM across all three TEOAE conditions were not significantly different except at 2.8 kHz, for which the mean AUCs for the power-weighted TEOAE condition (0.92) and TPP TEOAE condition (0.92) were significantly higher than the ambient TEOAE condition (0.87) (p=0.03 and p=0.02, respectively). There was no significant difference between AUCs for the power-weighted and TPP TEOAE conditions at any frequency for either SNR or CSM.
Discussion
Mean SNR and CSM values from the power-weighted TEOAE condition were significantly larger than mean values from the ambient and TPP conditions at most frequencies. The SNR and CSM of the power-weighted TEOAEs were largest (>15 dB for SNR) at 4 and 5.7 kHz for the normal hearing group, and larger than for the other two stimulus conditions. However, the power-weighted TEOAE stimulus also increased the TEOAE response in the SNHL group at these frequencies, so this did not significantly improve the ability based on AUC to identify SNHL relative to the other TEOAE conditions (see also Figure 8).
The mean SNR values for the SNHL group were generally below 4 dB across frequency for all TEOAE conditions (see Figure 5), yet notable exceptions to this were the mean power-weighted responses at 1, 4, and 5.7 kHz, at which the mean SNRs ranged between 6–9 dB (albeit with smaller median SNRs). In fact, the mean TEOAE SNRs for all three stimulus conditions were ≥5.8 dB at 5.7 kHz for the SNHL group. These features present in the mean SNR data appeared to influence AUC analyses inasmuch as performance at 4 and 5.7 kHz was not markedly better for the power-weighted TEOAE stimulus than for the other two TEOAE stimuli, and performance was clearly the poorest for all three TEOAE conditions at 5.7 kHz (AUC = 0.78 to 0.80) relative to the other half-octave frequencies.
It is possible to more closely examine results contributing to the relatively lower AUCs obtained at 5.7 kHz by examining Table 3. Table 3 provides the mean TEOAE signal and noise levels obtained for each group at each half-octave frequency. Each of these levels is the half-octave average of the band sound exposure spectrum level, termed SEL spectrum, defined with a reference averaging time of 1 s (Keefe et al. 2016). The mean TEOAE noise levels for both hearing groups at 5.7 kHz were similar across stimulus conditions, and were similar to the noise levels obtained at the adjacent frequencies of 4 and 8 kHz. Unlike other test frequencies, however, the mean signal level for the SNHL group at 5.7 kHz was approximately 6–8 dB greater than the mean noise level, whereas the difference between signal and noise levels was only about 3–4 dB at other frequencies. The mean signal and noise levels for both hearing groups were not the same across frequency (higher in the low frequencies) and yet the relative difference between signal and noise for the SNHL group remained fairly constant except at 5.7 kHz. It remains to be explained why the mean signal level for the SNHL group (see also Figure 5) was relatively greater than the mean noise level at 5.7 kHz compared to all other test frequencies evaluated in this study.
TABLE 3.
The mean (±1 SE) half-octave average of the SEL spectrum levels of the TEOAE signal and noise for each of the three TEOAE stimulus conditions: Ambient, TPP, and Power Weighted.
| Normal Hearing Group: | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| Frequency (kHz): | 1 | 2 | 2.8 | 4 | 5.7 | 8 |
|
|
||||||
| Ambient Signal (dB): | −21.7 (±0.5) | −29.6 (±0.5) | −32.4 (±0.6) | −34.3 (±0.6) | −33.6 (±0.7) | −29.8 (±0.8) |
| Ambient Noise (dB): | −34.6 (±0.3) | −38.5 (±0.2) | −43.1 (±0.2) | −47.6 (±0.3) | −45.9 (±0.3) | −43.5 (±0.3) |
|
|
||||||
| TPP Signal (dB): | −21.8 (±0.5) | −29.6 (±0.5) | −32.3 (±0.6) | −34.5 (±0.6) | −33.9 (±0.6) | −29.9 (±0.8) |
| TPP Noise (dB): | −34.4 (±0.3) | −38.1 (±0.2) | −43.0 (±0.2) | −47.5 (±0.3) | −46.0 (±0.3) | −43.3 (±0.3) |
|
|
||||||
| Power Weighted Signal (dB): | −19.8 (±0.5) | −28.1 (±0.4) | −30.4 (±0.5) | −31.4 (±0.6) | −30.8 (±0.6) | −30.8 (±0.7) |
| Power Weighted Noise (dB): | −35.0 (±0.3) | −39.0 (±0.2) | −43.7 (±0.2) | −48.4 (±0.3) | −46.9 (±0.3) | −43.8 (±0.2) |
|
|
||||||
| SNHL Group: | ||||||
|
|
||||||
| Frequency (kHz): | 1 | 2 | 2.8 | 4 | 5.7 | 8 |
|
|
||||||
| Ambient Signal (dB): | −29.3 (±1.5) | −34.2 (±0.9) | −39.4 (±0.7) | −42.8 (±0.7) | −38.9 (±0.7) | −38.1 (±0.5) |
| Ambient Noise (dB): | −34 (±0.8) | −37.9 (±0.5) | −42.4 (±0.4) | −46.8 (±0.4) | −44.9 (±0.4) | −41.9 (±0.3) |
|
|
||||||
| TPP Signal (dB): | −28.8 (±1.7) | −34.8 (±0.9) | −39.6 (±0.7) | −43.2 (±0.8) | −38.9 (±0.7) | −38.3 (±0.5) |
| TPP Noise (dB): | −33.6 (±1.4) | −37.7 (±0.6) | −42.0 (±0.4) | −46.7 (±0.4) | −44.6 (±0.3) | −42.1 (±0.3) |
|
|
||||||
| Power Weighted Signal (dB): | −26.0 (±1.9) | −33.6 (±0.8) | −38.9 (±0.6) | −41.4 (±0.9) | −37.3 (±0.8) | −38.1 (±0.5) |
| Power Weighted Noise (dB): | −32.2 (±1.6) | −37.5 (±0.4) | −42.5 (±0.3) | −47.7 (±0.4) | −45.7 (±0.3) | −42.0 (±0.3) |
|
|
||||||
One finding described in Keefe et al. (2016) is that when distortion was initially observed with this probe, it typically occurred around 5 kHz. If present, the origin of the distortion level observed at 5.7 kHz in the SNHL group may have been associated with probe distortion rather than having a biological origin. As described in the methods, approximately 20% of both normal-hearing and SNHL ears received TEOAE tests using a stimulus bandwidth up to 10 kHz, while the other 80% of ears received TEOAE tests using a stimulus bandwidth up to 8 kHz. All TEOAE data in the present study were performed over the same analysis bandwidth up to 8 kHz. This was based on preliminary analyses, which showed the presence of distortion in some ears between 8 and 10 kHz. Those preliminary results were the reason that the maximum stimulus frequency was reduced from 10 to 8 kHz in testing most of the ears. The presence of elevated TEOAE SNR and CSM at 5.7 kHz in the group of SNHL ears may have been due to the inclusion of test results using the stimulus bandwidth up to 10 kHz. The probe used in this study had the advantages of accurate reflectance and admittance measurements. These advantages made possible the studies of TEOAEs in the pressurized ear canal and TEOAE measurements using a weighted stimulus with approximately constant absorbed sound power across frequency. Despite these advantages, the tendency for this probe to generate some distortion in TEOAEs at 5.7 kHz was a limitation to this study.
The overall AUC findings from this study do suggest that WB TEOAEs, particularly when power-weighted, show good performance at identifying ears with SNHL at half-octave frequencies from 1 to 8 kHz with AUCs of 0.85 to 0.93 for most TEOAE conditions across this range. The power-weighted condition and, to a lesser extent, the TPP TEOAE condition were superior to the ambient TEOAE condition at identifying SNHL, although this performance difference was only significant in the mid-frequencies (2 and 2.8 kHz), and trends were in the opposite direction at 1 and 8 kHz. All TEOAE conditions performed similarly for differentiating SNHL from normal hearing, with average AUCs for SNR across audiometric frequencies of 0.85 for the ambient condition, 0.86 for the TPP condition, and 0.87 for the power-weighted condition. The unweighted TEOAE stimulus condition had an approximately constant level of incident sound pressure across frequency, whereas the power-weighted condition had a constant level of absorbed sound power across frequency. As described in Keefe et al. (2016) and Keefe et al. (2017), both of these TEOAE stimulus calibrations controlled for acoustic standing-wave effects in the ear canal. In this context, the similar test performance of TEOAEs in the ambient and power-weighted stimulus conditions in classifying ears with SNHL might be expected.
These study findings on TEOAE performance (except at 5.7 kHz) are in substantial agreement with previous studies that addressed the ability of OAE measurements to accurately distinguish ears with SNHL from those with normal hearing. Gorga et al. (1993) measured TEOAEs from 0.5 to 4 kHz in children and adult participants, with results suggesting that TEOAE performance was most accurate at 1 and 2 kHz (AUC = 0.92 and 0.93). Goodman et al. (2009) explored the relationship between behavioral hearing thresholds and click-evoked OAEs, recording measurable OAEs up to 15.0 kHz in some ears. Keefe et al. (2011) further analyzed the OAE data collected by Goodman and colleagues to measure its accuracy at detecting high-frequency hearing losses, and reported a mean AUC of 0.90 for measurements obtained from 1 to 10.1 kHz. Their AUC findings for TEOAEs were consistent with previous studies at TEOAE frequencies up to 4 kHz (e.g., Prieve et al. 1993; Lichtenstein & Stapells 1996; Hussain et al. 1998), and all of these were relatively consistent with the AUC values obtained in the present study.
In the current study, mean SNR reliability values were generally between 2–4 dB (and less for the SNHL group across most frequencies), with standard errors well below ±0.5 dB for both of the hearing groups for all three TEOAE conditions. The reliability and performance findings (based on AUC) from this study in combination are initially promising, despite that the size of the dataset is relatively modest and the findings have yet to be replicated. The results justify that a continued exploration of TEOAE measurements for monitoring hearing status is warranted to further examine differences in test reliability and test performance with the stimulus and other testing conditions (i.e., ambient-pressure versus TPP testing). Such future studies may include TEOAE and reflectance testing in ears that may have permanent or intermittent conductive disorders, to examine the ability of these tests to classify ears with differing types of hearing loss (SNHL, conductive or mixed).
The reliability of TEOAE measurements in tests repeated one to two months apart was similar to the reliability reported in previous distortion product otoacoustic emission (DPOAE) and TEOAE studies (e.g., Franklin et al. 1992; Dreisbach et al. 2006; Keefe et al. 2011). Dreisbach and colleagues reported a mean reliability of 2.8 dB (SD = ±2.7) for DPOAE f2 levels from 2 to 8 kHz, and 5.15 dB (SD = ±4.4) for levels from >8 to 16 kHz, while Keefe and colleagues reported median click-evoked OAE test-retest differences of <4 dB from 0.5 to 12.7 kHz. Data from Dreisbach and colleagues also revealed that reliability appeared to generally increase across frequency, as reliability grew from about 2 dB to 4–5 dB from 2 to 8 kHz. The same trend appeared to be true for Keefe and colleagues, although this only appeared to be the case for findings from 4 to 16 kHz, whereas there was an essentially flat trend across lower frequencies.
Previous studies measured repeatability of OAEs across periods of days and/or weeks in normal hearing participants for the purpose of monitoring cochlear status (e.g., Franklin et al. 1992; Roede et al. 1993; Dreisbach et al. 2006). These studies provided results for OAEs collected up to 8 kHz. Findings were also obtained at higher test frequencies from 10–16 kHz in Dreisbach et al. (2006). Depending on the study, mean OAE level reliability varied anywhere from 1 to 5 dB across measurement intervals for conventional test frequencies (≤8 kHz), and reliability tended to increase for higher-frequency measurements (>8 kHz). A potential limitation to these studies was that middle-ear function was only characterized by the presence or absence of air-bone gaps and 226-Hz tympanometry, whereas the present study used a middle-ear test that assesses status across the frequency range of OAE measurement.
There are several potential limitations of the present study to address. One limitation is with regard to the SNHL group, as there were fewer numbers to contrast to the normal-hearing group at lower test frequencies (see Table 1). For example, there were only 16 and 22 ears in the SNHL group compared to 140 and 134 ears in the normal-hearing group at 1 and 2 kHz, respectively, which are low proportions. This means that statistical contrasts between normal-hearing and SNHL groups performed at these frequencies should be interpreted with caution, although findings at these frequencies may be less critical for the purpose of monitoring for initial hearing loss given that SNHL typically originates in higher frequency regions. This limitation may, however, be of less consequence if there is ultimately more interest in OAE performance at higher frequencies to monitor for changes in high-frequency thresholds, as would be the case with ototoxicity monitoring.
An additional limitation is that it was necessary to compare half-octave TEOAEs with the nearest audiometric test frequency, and therefore audiometric and TEOAE findings may not be wholly reflective of the status in a precisely similar region of the cochlea. This appears to be a minor limitation inasmuch as TEOAE measures were averaged over half octaves centered at 2.8 and 5.7 kHz in order to classify ears as normal or having a SNHL based on audiometric frequencies of 3 and 6 kHz. The latter pair of frequencies were within 7% of the TEOAE center frequency.
Also of note as a feature more so than a limitation, the mean peSPL of the TEOAE stimulus was almost identical between the three TEOAE conditions but the overall mean SPL from the power-weighted TEOAE stimulus was about 2 dB higher than the other two conditions (see Table 2). It is possible that this difference may have accounted for some of the differences observed between the power-weighted TEOAE condition and the other two TEOAE conditions. The overall calibration of the power-weighted TOAE stimulus was based on a brief measurement of peSPL (7.5 s) prior to the main data recording (data in this brief measurement were subsequently discarded, while the DAC gains were adjusted to produce the desired peSPL), whereas the mean peSPL reported here was calculated for TEOAE data obtained over the main one-minute recording. This shorter stimulus duration for the in-the-ear calibration of peSPL is a source of noise that contributed to any differences in mean peSPL between stimulus conditions.
In regard to future efforts, given the similar ability of all three TEOAE test types to reliably identify SNHL from normal hearing in subjects with clinically normal tympanometry and no audiometric conductive component, a subsequent approach would be to evaluate these test types in clinical populations that may have a greater disparity in middle-ear pressure and/or large variations in absorbance as a function of frequency. It would also be of interest to evaluate OAEs evoked by a chirp that is power-weighted in a test in which the ear canal was pressurized to the individual TPP in each ear. This approach would be of particular interest by testing adult clinical groups where variability in TPP is common, or in newborns receiving a newborn hearing screening exam to determine if such TEOAE testing would reduce the false-positive screening rates in identifying hearing loss. While there was little difference in the present adult study between TEOAE results in the unweighted ambient-pressure and TPP stimulus conditions, this result may have been due to the inclusion criterion for middle-ear pressure. The TPP distribution in Figure 9 reveals that approximately 90% of ears had a TPP within ±20 daPa.
Figure 9.
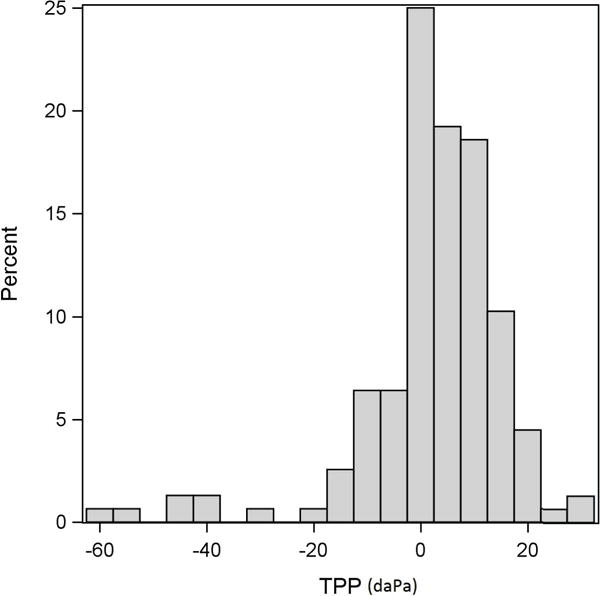
The distribution of TPP (of the downswept tympanogram) from the first valid session for all of the ears (normal hearing and SNHL).
Conclusion
TEOAE performance elicited with a chirp stimulus was evaluated in an ambient condition, at TPP and in a power-weighted stimulus condition that accounted for individual differences in middle-ear function. Ears were included in the study with normal middle-ear static acoustic admittance values and a TPP in the normal range. All three conditions showed good performance (with AUCs >0.85) in identifying ears with SNHL at frequencies between 1 and 8 kHz, except for lower performance at 5.7 kHz. Further research is needed to evaluate the performance of the three TEOAE test conditions in patients with more varied middle-ear function, including variations in middle-ear pressure and WB absorbance.
Acknowledgments
All authors contributed equally to this work. MPF, DHK and LLH collaborated on study design, and DHK and DFF developed software for data collection and participated in data reduction and analysis. ACG and DBP engaged in data collection, MPF and DBP also analyzed data, and DBP wrote the paper. GPM developed statistical models of the data. All authors discussed the results and implications and commented on the manuscript at all stages.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the US Department of Veterans Affairs.
Footnotes
Conflicts of Interest and Sources of Funding:
D. H. Keefe is involved in commercializing new technology for screening and diagnosis of middle-ear and cochlear disorders. This research was funded by the National Institute on Deafness and other Communication Disorders (NIDCD) of the National Institutes of Health (NIH) under award numbers DC10202 and DC004662. This work was also supported with resources and the use of facilities at the VA Portland Health Care System, VA RR&D NCRAR Center award C9230C.
References
- ANSI S3.6. Specifications for Audiometers. New York, NY: American National Standards Institute; 2010. [Google Scholar]
- ANSI S3.39. Specifications for Instruments to Measure Aural Acoustic Impedance and Admittance (Aural Acoustic Immittance) (ANSI S3.39–1987, R2012) New York, NY: American National Standards Institute; 2012. [Google Scholar]
- Bennett CL, Ozdamar O. High-frequency transient evoked otoacoustic emissions acquisition with auditory canal compensated clicks using swept-tone analysis. J Acoust Soc Am. 2010a;127:2410–2419. doi: 10.1121/1.3279831. [DOI] [PubMed] [Google Scholar]
- Bennett CL, Ozdamar O. Swept-tone transient-evoked otoacoustic emissions. J Acoust Soc Am. 2010b;128:1833–1844. doi: 10.1121/1.3467769. [DOI] [PubMed] [Google Scholar]
- DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- Dreisbach LE, Long KM, Lees SE. Repeatability of high-frequency distortion-product otoacoustic emissions in normal-hearing adults. Ear Hear. 2006;27:466–479. doi: 10.1097/01.aud.0000233892.37803.1a. [DOI] [PubMed] [Google Scholar]
- Ellison JC, Keefe DH. Audiometric predictions using stimulus-frequency otoacoustic emissions and middle ear measurements. Ear Hear. 2005;26:487–503. doi: 10.1097/01.aud.0000179692.81851.3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeney MP, Hunter LL, Kei J, et al. Consensus statement: Eriksholm workshop on wideband absorbance measures of the middle ear. Ear Hear. 2013;34:78S–79S. doi: 10.1097/AUD.0b013e31829c726b. [DOI] [PubMed] [Google Scholar]
- Feeney MP, Keefe DH, Hunter LL, et al. Normative wideband reflectance, equivalent admittance at the tympanic membrane, and acoustic stapedius reflex threshold in adults. Ear Hear. 2016 Dec 30; doi: 10.1097/AUD.0000000000000399. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeney MP, Sanford CA, Putterman DB. Effects of ear-canal static pressure on puretone thresholds and wideband acoustic immittance. J Am Acad Audiol. 2014a;25:462–470. doi: 10.3766/jaaa.25.5.5. [DOI] [PubMed] [Google Scholar]
- Feeney MP, Stover B, Keefe DH, et al. Sources of variability in wideband energy reflectance measurements in adults. J Am Acad Audiol. 2014b;25:449–461. doi: 10.3766/jaaa.25.5.4. [DOI] [PubMed] [Google Scholar]
- Franklin DJ, McCoy MJ, Martin GK, et al. Test/retest reliability of distortion-product and transiently evoked otoacoustic emissions. Ear Hear. 1992;13:417–429. doi: 10.1097/00003446-199212000-00008. [DOI] [PubMed] [Google Scholar]
- Goodman SS, Fitzpatrick DF, Ellison JC, et al. High-frequency click-evoked otoacoustic emissions and behavioral thresholds in humans. J Acoust Soc Am. 2009;125:1014–1032. doi: 10.1121/1.3056566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorga MP, Neely ST, Bergman B, et al. Otoacoustic emissions from normal-hearing and hearing-impaired subjects: distortion product responses. J Acoust Soc Am. 1993;93:2050–2060. doi: 10.1121/1.406691. [DOI] [PubMed] [Google Scholar]
- Gorga MP, Neely ST, Ohlrich B, et al. From laboratory to clinic: a large scale study of distortion product otoacoustic emissions in ears with normal hearing and ears with hearing loss. Ear Hear. 1997;18:440–455. doi: 10.1097/00003446-199712000-00003. [DOI] [PubMed] [Google Scholar]
- Hussain DM, Gorga MP, Neely ST, et al. Transient evoked otoacoustic emissions in patients with normal hearing and in patients with hearing loss. Ear Hear. 1998;19:434–449. doi: 10.1097/00003446-199812000-00005. [DOI] [PubMed] [Google Scholar]
- Jedrzejczak WW, Kochanek K, Sliwa L, et al. Chirp-evoked otoacoustic emissions in children. Int J Pediatr Otorhinolaryngol. 2013;77:101–106. doi: 10.1016/j.ijporl.2012.10.005. [DOI] [PubMed] [Google Scholar]
- Kalluri R, Shera CA. Measuring stimulus-frequency otoacoustic emissions using swept tones. J Acoust Soc Am. 2013;134:356–368. doi: 10.1121/1.4807505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe DH. Double-evoked otoacoustic emissions: I, Measurement theory and nonlinear coherence. J Acoust Soc Am. 1998;103:3489–3498. doi: 10.1121/1.423058. [DOI] [PubMed] [Google Scholar]
- Keefe DH. Influence of middle-ear function and pathology on otoacoustic emissions. In: Robinette MRG, J T, editors. Otoacoustic Emissions: Clinical Applications. 3rd. New York: Thieme; 2007. pp. 163–196. [Google Scholar]
- Keefe DH. Moments of click-evoked otoacoustic emissions in human ears: group delay and spread, instantaneous frequency and bandwidth. J Acoust Soc Am. 2012;132:3319–3350. doi: 10.1121/1.4757734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe DH, Feeney MP, Hunter LL, et al. Comparisons of transient evoked otoacoustic emissions using chirp and click stimuli. J Acoust Soc Am. 2016;140:1949–1973. doi: 10.1121/1.4962532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe DH, Feeney MP, Hunter LL, et al. Comparing otoacoustic emissions evoked by chirp transients with constant absorbed sound power and constant incident pressure magnitude. J Acoust Soc Am. 2017;141:499–514. doi: 10.1121/1.4974146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe DH, Goodman SS, Ellison JC, et al. Detecting high-frequency hearing loss with click-evoked otoacoustic emissions. J Acoust Soc Am. 2011;129:245–261. doi: 10.1121/1.3514527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe DH, Hunter LL, Patrick Feeney M, et al. Procedures for ambient-pressure and tympanometric tests of aural acoustic reflectance and admittance in human infants and adults. J Acoust Soc Am. 2015;138:3625–3653. doi: 10.1121/1.4936946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe DH, Ling R. Double-evoked otoacoustic emissions. II. Intermittent noise rejection, calibration and ear-canal measurements. J Acoust Soc Am. 1998;103:3499–3508. doi: 10.1121/1.423058. [DOI] [PubMed] [Google Scholar]
- Keefe DH, Schairer KS. Specification of absorbed-sound power in the ear canal: application to suppression of stimulus frequency otoacoustic emissions. J Acoust Soc Am. 2011;129:779–791. doi: 10.1121/1.3531796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe DH, Simmons JL. Energy transmittance predicts conductive hearing loss in older children and adults. J Acoust Soc Am. 2003;114:3217–3238. doi: 10.1121/1.1625931. [DOI] [PubMed] [Google Scholar]
- Konradsson KS, Svensson O, Carlborg B, et al. Tympanic pressure gradients and otoacoustic emissions. Ear Hear. 1999;20:403–409. doi: 10.1097/00003446-199910000-00003. [DOI] [PubMed] [Google Scholar]
- Lichtenstein V, Stapells DR. Frequency-specific identification of hearing loss using transient-evoked otoacoustic emissions to clicks and tones. Hear Res. 1996;98:125–136. doi: 10.1016/0378-5955(96)00084-6. [DOI] [PubMed] [Google Scholar]
- Liu YW, Sanford CA, Ellison JC, et al. Wideband absorbance tympanometry using pressure sweeps: system development and results on adults with normal hearing. J Acoust Soc Am. 2008;124:3708–3719. doi: 10.1121/1.3001712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonsbury-Martin BL, Whitehead ML, Martin GK. Clinical applications of otoacoustic emissions. J Speech Hear Res. 1991;34:964–981. doi: 10.1044/jshr.3405.964. [DOI] [PubMed] [Google Scholar]
- Marshall L, Heller LM, Westhusin LJ. Effect of negative middle-ear pressure on transient-evoked otoacoustic emissions. Ear Hear. 1997;18:218–226. doi: 10.1097/00003446-199706000-00005. [DOI] [PubMed] [Google Scholar]
- Marshall L, Lapsley Miller JA, Guinan JJ, et al. Otoacoustic-emission-based medial-olivocochlear reflex assays for humans. J Acoust Soc Am. 2014;136:2697–2713. doi: 10.1121/1.4896745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan GP. On reliability. Ear Hear. 2014;35:589–590. doi: 10.1097/AUD.0000000000000042. [DOI] [PubMed] [Google Scholar]
- McMillan GP, Reavis KM, Konrad-Martin D, et al. The statistical basis for serial monitoring in audiology. Ear Hear. 2013;34:610–618. doi: 10.1097/AUD.0b013e31828a21b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeve SL, Margolis RH, Levine SC, et al. Effect of ear-canal pressure on evoked otoacoustic emissions. J Acoust Soc Am. 1992;91:2091–2095. doi: 10.1121/1.403695. [DOI] [PubMed] [Google Scholar]
- Neumann J, Uppenkamp S, Kollmeier B. Chirp evoked otoacoustic emissions. Hear Res. 1994;79:17–25. doi: 10.1016/0378-5955(94)90123-6. [DOI] [PubMed] [Google Scholar]
- Osterhammel PA, Nielsen LH, Rasmussen AN. Distortion product otoacoustic emissions: the influence of the middle ear transmission. Scand Audiol. 1993;22:111–116. doi: 10.3109/01050399309046026. [DOI] [PubMed] [Google Scholar]
- Owens JJ, McCoy MJ, Lonsbury-Martin BL, et al. Otoacoustic emissions in children with normal ears, middle ear dysfunction, and ventilating tubes. Am J Otol. 1993;14:34–40. [PubMed] [Google Scholar]
- Plinkert PK, Bootz F, Vossieck T. Influence of static middle ear pressure on transiently evoked otoacoustic emissions and distortion products. Eur Arch Otorhinolaryngol. 1994;251:95–99. doi: 10.1007/BF00179900. [DOI] [PubMed] [Google Scholar]
- Prieve BA, Calandruccio L, Fitzgerald T, et al. Changes in transient-evoked otoacoustic emission levels with negative tympanometric peak pressure in infants and toddlers. Ear Hear. 2008;29:533–542. doi: 10.1097/AUD.0b013e3181731e3e. [DOI] [PubMed] [Google Scholar]
- Prieve BA, Gorga MP, Schmidt A, et al. Analysis of transient-evoked otoacoustic emissions in normal-hearing and hearing-impaired ears. J Acoust Soc Am. 1993;93:3308–3319. doi: 10.1121/1.405715. [DOI] [PubMed] [Google Scholar]
- Reavis KM, McMillan GP, Dille MF, et al. Meta-analysis of distortion product otoacoustic emission retest variability for serial monitoring of cochlear function in adults. Ear Hear. 2015;36:251–260. doi: 10.1097/AUD.0000000000000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roede J, Harris FP, Probst R, et al. Repeatability of distortion product otoacoustic emissions in normally hearing humans. Audiology. 1993;32:273–281. doi: 10.3109/00206099309072943. [DOI] [PubMed] [Google Scholar]
- Sanford CA, Hunter LL, Feeney MP, et al. Wideband acoustic immittance: tympanometric measures. Ear Hear. 2013;34(Suppl 1):65S–71S. doi: 10.1097/AUD.0b013e31829c7250. [DOI] [PubMed] [Google Scholar]
- Shaver MD, Sun XM. Wideband energy reflectance measurements: effects of negative middle ear pressure and application of a pressure compensation procedure. J Acoust Soc Am. 2013;134:332–341. doi: 10.1121/1.4807509. [DOI] [PubMed] [Google Scholar]
- Shera CA, Cooper NP. Basilar-membrane interference patterns from multiple internal reflection of cochlear traveling waves. J Acoust Soc Am. 2013;133:2224–2239. doi: 10.1121/1.4792129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun XM. Ear canal pressure variations versus negative middle ear pressure: comparison using distortion product otoacoustic emission measurement in humans. Ear Hear. 2012;33:69–78. doi: 10.1097/AUD.0b013e3182280326. [DOI] [PubMed] [Google Scholar]
- Sun XM, Shaver MD. Effects of negative middle ear pressure on distortion product otoacoustic emissions and application of a compensation procedure in humans. Ear Hear. 2009;30:191–202. doi: 10.1097/AUD.0b013e31819769e1. [DOI] [PubMed] [Google Scholar]
- Trine MB, Hirsch JE, Margolis RH. The effect of middle ear pressure on transient evoked otoacoustic emissions. Ear Hear. 1993;14:401–407. doi: 10.1097/00003446-199312000-00005. [DOI] [PubMed] [Google Scholar]