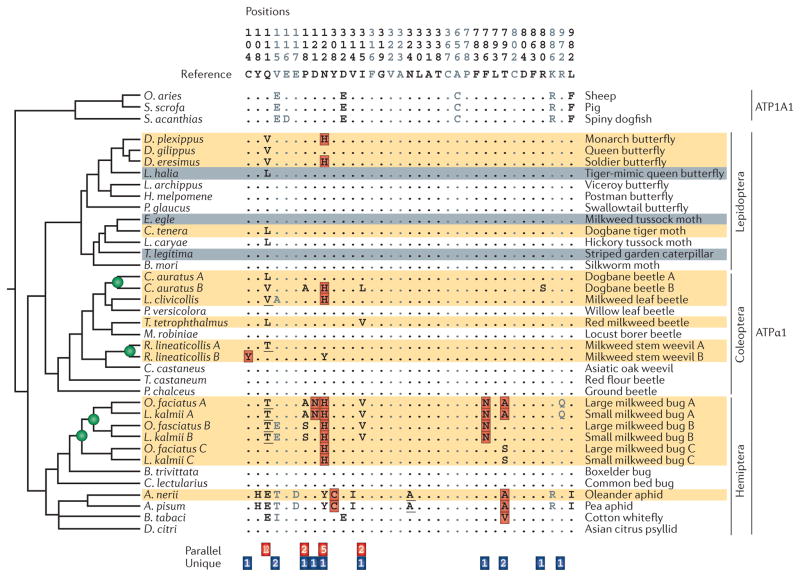
Figure 2. Phylogenetic patterns of parallel and unique substitutions in ATPα1 that are associated with resistance to toxic cardenolides in herbivorous insects.
Substitutions at the indicated sites in Na+,K+-ATPase α1 subunit (ATPα1) are implicated in cardenolide-binding in insect taxa that feed on Apocynaceae. Names of insect orders are shown on the far right. Numbered columns correspond to sites that are implicated in cardenolide-binding based on experimental evidence. Grey sites correspond to sites that may have a role in cardenolide-binding based on structural considerations. Dots denote identity with the consensus sequence. Outgroup sequences of vertebrate Na+,K+-ATPase α1 subunit (ATP1A1; a homologue of ATPα1) from several taxa (sheep, pig and spiny dogfish) are shown for reference. Letters in red boxes represent amino acid replacements whose functional effects have been experimentally characterized. Underlined letters are substitutions that require at least two nonsynoymous substitutions relative to the ancestral sequence. Orange-shaded rows correspond to specialists that are known to sequester cardenolides. Grey-shaded rows represent species that either are not known to sequester cardenolides and/or are nonspecialists that are only occasionally found on Apocynaceae. Green circles represent inferred duplications of the gene encoding ATPα1. Numbers at the bottom of the figure correspond to the number of inferred substitutions associated with use of Apocynaceae. Red boxes at the bottom of the figure correspond to parallel substitutions (observed in more than one independent lineage). Blue boxes correspond to unique substitutions (observed in only one lineage). Note that a preponderance of parallel substitutions occurs only in taxa that possess a single copy of the gene encoding ATPα1. In those taxa that possess two duplicate copies, a greater number of unique (nonshared) substitutions have occurred. It may be that the possession of two functionally redundant paralogues alleviates pleiotropic constraints, so a broader spectrum of function-altering mutations can contribute to adaptation. A. nerii, Aphis nerii; A. pisum, Acyrthosiphon pisum; B. mori, Bombyx mori; B. tabaci, Bemisia tabaci; B. trivittata, Boisea trivittata; C. auratus, Chrysochus auratus; C. castaneus, Cyrtepistomus castaneus; C. lectularius, Cimex lectularius; C. tenera, Cycnia tenera; D. citri, Diaphorina citri; D. eresimus, Danaus eresimus; D. gilippus, Danaus gilippus; D. plexippus, Danaus plexippus; E. egle, Euchaetes egle; H. melpomene, Heliconius melpomene; L. archippus, Limenitis archippus; L. caryae, Lophocampa caryae; L. clivicollis, Labidomera clivicollis; L. halia, Lycorea halia; L. kalmii, Lygaeus kalmii; M. robiniae, Megacyllene robiniae; O. aries, Ovis aries; O. faciatus, Oncopeltus fasciatus; P. chalceus, Pogonus chalceus; P. glaucus, Papilio glaucus; P. versicolora, Plagiodera versicolora; R. lineaticollis, Rhyssomatus lineaticollis; S. acanthias, Squalus acanthias; S. scrofa, Sus scrofa; T. castaneum, Tribolium castaneum; T. legitima, Trichordestra legitima; T. tetrophthalmus, Tetraopes tetrophthalmus. Adapted from Zhen, Y., Aardema, M. L., Medina, E. M., Schumer, M. & Andolfatto, P. Parallel molecular evolution in an herbivore community. Science 337, 1634–1637 (2012). Reprinted with permission from the American Association for the Advancement of Science.