Figure 2.
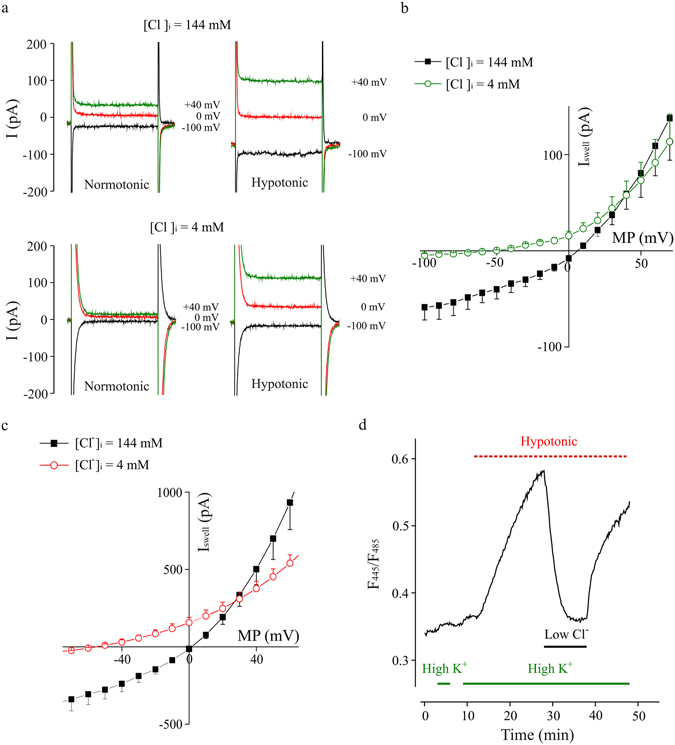
Hypotonic-activated current is driven by Cl− ions, in microglia cells. (a) Representative whole-cell currents recorded under acute application of the hypotonic extracellular solution (8–10% dilution), at the indicated membrane potentials, with a standard ([Cl−]i = 144 mM; upper traces) or a low chloride ([Cl−]i = 4 mM; lower traces) pipette solution. As indicated, the left traces were recorded in normotonic condition, just before applying the hypotonic medium; the right traces were recorded 21 minutes after hypotonic stimulation. (b) Current-voltage relationships of the currents recorded in control condition ([Cl−]i = 144 mM; black squares; n = 3) and with a low chloride pipette solution ([Cl−]i = 4 mM; green circles; n = 4; HP = −70 mV) during acute application of hypotonic medium. Note the shift of the current reversal potential to more negative values. (c) Current-voltage relationships recorded in BV-2 cells, exposed acutely to a hypotonic medium (205 mOsm), in control condition ([Cl−]i = 144 mM; black squares, n = 9) and lowering Cl− concentration ([Cl−]i = 4 mM; red circles; n = 7) in the pipette solution. Graph represents the swell-activated current (ISwell; subtraction of current in control to that under hypotonic stimulation) to eliminate the overlap with endogenous cell conductances. Note the shift of the current reversal potential to more negative values. (d) Time course of fluorescence ratio increase induced by the exposure of BV-2 cells, transfected with Cl− Sensor, to a hypotonic solution (200 mOsm, red bar) in normal or low extracellular Cl− (6 mM, black bar). Cells are concomitantly depolarized by high K+ extracellular medium (60 mM, green bar), which per se did not cause significant changes in the fluorescence ratio.
