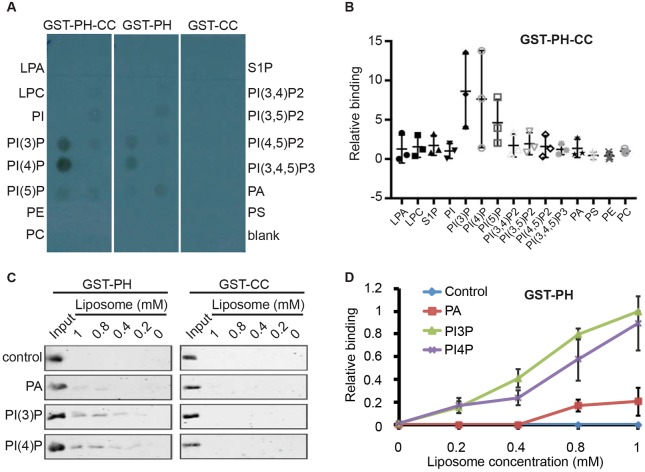
Fig. 5.
The PH domain of TBC-2 binds PI3P and PI4P in vitro. (A) Protein–lipid overlay assay demonstrating that the PH-CC and PH domains, but not the CC domain alone, of TBC-2 bind to PI(3)P and PI(4)P. (B) The relative binding of GST::PH-CC to each lipid as compared to the blank from three independent protein–lipid overlay experiments as assessed with ImageJ. Bars show the mean and range for the three data points. (C) The binding between purified recombinant GST::PH and GST::CC proteins, and liposomes containing different phospholipids were detected by western blotting using an anti-GST antibody. The control liposomes were composed of PC and PE. The PA, PI(3)P and PI(4)P liposomes were composed of PC and PE, with either PA, PI(3)P or PI(4)P, respectively. (D) Quantification of GST::PH lipid-binding results in C. The bindings to different liposomes were normalized to the input. The results are mean±s.d. from three independent experiments. LPA, lysophosphatidic acid; LPC, lysophosphocholine; PE, phosphatidylethanolamine; PC, phosphatidylcholine; S1P, sphingosine-1-phosphate; PA, phosphatidic acid; PS, phosphatidylserine.