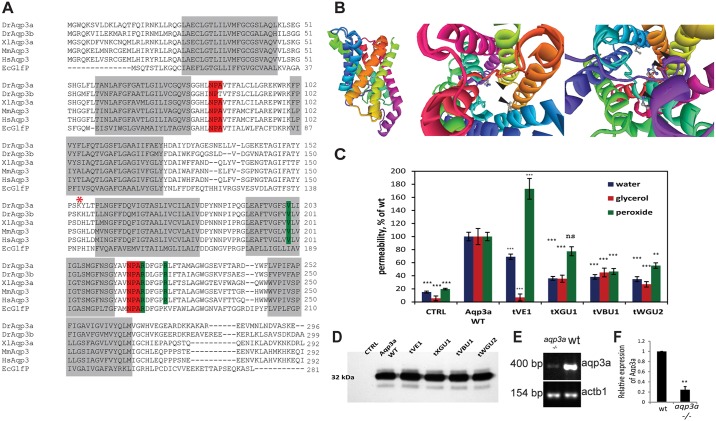
Fig. 2.
Mutations in aqp3a alter pore permeability. (A) Alignment of Aqp3a amino acid sequence with zebrafish paralogue Aqp3b and homologues from human (Hs), mouse (Mm), X. laevis (Xl), and E. coli (Ec). The transmembrane domains and the conserved NPA motifs of the water selectivity filter are highlighted in grey and red, respectively. Mutated amino acid residues in the dominant mau alleles are highlighted in green. The start of the frameshift in the loss-of-function allele is marked with an asterisk. (B) Homology-based model of zebrafish Aqp3a. (Left) Side view of the aquaporin monomer. (Middle) View from the cytoplasmic side of the channel, emphasizing mutated amino acids. (Right) View from the extracellular side of the channel. Arrowheads point to the mutated amino acids in the pore surface of the protein. (C) Mutant variants of Aqp3a have altered permeability for water, glycerol and peroxide when expressed in Xenopus oocytes. CTRL, uninjected oocytes. Solute and allele data were obtained from 11-12 oocytes and normalized to wild type. Mean±s.e.m. ***P<0.001, **P<0.01; ns, not statistically significant; wild-type and mutant aquaporin variants versus uninjected oocytes for each cargo (unpaired t-test). (D) Western blot showing expression levels of wild-type and mutant zebrafish Aqp3a in Xenopus oocytes. CTRL, water-injected oocytes. Total membranes from ten oocytes were pooled and equivalent membrane extract from two oocytes was loaded on the gel. (E) Semi-quantitative RT-PCR showing reduced abundance of aqp3a transcript in aqp3a−/− compared with the wild-type sibling. actb1 was used as a control. (F) The intensity of the aqp3a band in E was normalized to the actb1 control to compare wild type and aqp3a−/−. n=3. **P<0.01, paired t-test.