Summary
Objective
Minimally invasive surgery (MIS) techniques are anecdotally reported to be increasingly used, but little objective data supports this. Our objective was to assess trends in MIS utilization across various procedures in pediatric urology and to compare postoperative complication rates between MIS and open procedures.
Methods
We analyzed the 1998–2012 Nationwide Inpatient Sample. We identified children (<18 years old) undergoing open and MIS inpatient procedures and any in-hospital postoperative complications that occurred during that postoperative hospitalization. We utilized propensity score matching and multivariable logistic regression to adjust for confounding factors.
Results
We identified 163,838 weighted encounters in the “overall cohort,” 70,273 of which were at centers performing more than five MIS procedures over the years studied. Use of MIS techniques increased significantly over time for several procedures, most prominently for nephrectomy (Figure). The overall rate of complications was lower in patients undergoing MIS compared with open surgery (6% vs. 11%, p<0.001). Specialized centers had a significantly lower overall rate of complications than unspecialized centers (9% vs. 12%, p<0.001). Within specialized centers, MIS had lower complication rates than open procedures (7% vs. 9%, p<0.001); this finding was consistent even after adjusting for other factors (OR 0.71, p=0.02).
Discussion
Limitations include that these data may not be generalizable to encounters not in the sample pool. As a large, retrospective, administrative database, NIS may be affected by miscoding bias – rendering our analysis sensitive to the accuracy of procedure coding in NIS. Although the accuracy level of NIS is high for an administrative database, it is possible at least some portion of our cohort may be incorrectly coded. Further, the NSQIP complications we identified may represent associated comorbidities and not true postoperative complications, as NIS does not provide temporal relationships between different diagnosis codes. Despite these limitations, we note that the NIS database is rigorously monitored and audited for coding accuracy and, therefore, represents a reasonably reliable panorama of the characteristics of an inpatient surgical cohort. However, it is important to note that the choice of operative modality is, undoubtedly, multifactorial and patient/setting-specific.
Conclusions
There is increasing use of MIS for pediatric urology procedures, although utilization rates vary among procedures. MIS was associated with a lower postoperative complication rate than for open procedures. Higher-volume MIS centers have a lower complication rate than lower-volume centers.
Keywords: Urology, Pediatrics, Laparoscopic surgery, Minimally invasive surgery, Complications
Introduction
Initially pioneered primarily as a diagnostic modality in the 1970s, the use of minimally invasive surgery (MIS) has become increasingly common in pediatric surgical practice following significant recent technological improvements [1–3]. Pediatric urologists have embraced MIS as technological improvements have come to market, altering the treatment landscape and permitting viable MIS approaches for many common urinary tract procedures in children [4]. This change has coincided with paradigm shifts among physicians and parents favoring less-invasive surgical techniques in children when possible, driven by reported improvements in cosmetic outcomes, more expedient postoperative discharge, and faster recovery times [5–8]. Extensive MIS training is now a component of most urological residency and fellowship programs in the USA, and urological procedures account for up to 15% of all laparoscopic operations performed in children domestically [9,10].
Use of MIS, however, is not without potential trade-offs. Both laparoscopy and robotic surgery require considerable resource expenditure in training and infrastructure [11,12]. Operative times for MIS are typically longer than comparable open approaches and are highly dependent on operator proficiency [13–15]. Yet, despite these substantial differences, studies directly comparing the outcomes from MIS and open approaches in pediatric urology are limited.
We sought to describe changes in the frequency of MIS use in pediatric urology in the USA over a 14-year period using a nationwide all-payer database and to characterize differences in the frequency of reported postoperative complications in MIS and open pediatric urological surgical approaches. We hypothesized that there would be an increase in both MIS utilization and MIS-related complications (because of individual provider learning curves) over time.
Methods
Data source
The Nationwide Inpatient Sample (NIS) is an all-payer database managed by the Healthcare Cost and Utilization Project (HCUP) and sponsored by the Agency for Healthcare Research and Quality. Data in the NIS are from a 20% stratified probability sample of US hospitals based on five hospital characteristics including ownership status, number of beds, teaching status, urban/rural location, and geographic region. NIS includes post-stratification discharge weights to estimate 35 million hospital admissions per year.
Selection of patients and covariates
We selected all pediatric patients (<18 years old) between 1998 and 2012 undergoing procedures which could reasonably be performed open or with MIS and defined this as the “entire cohort.” Procedures were defined by ICD-9-CM code (see Appendix 1 for codes and procedures); codes were previously validated at our institution to assure their accuracy [16,17]. We defined MIS procedures as those with a concurrent ICD-9-CM procedure code for laparoscopic/robotic assistance (54.51, 54.21, and/or 17.4x) [18–21]. We then refined our cohort to compare only hospitals that performed a minimum of five MIS cases per year to compare hospitals where enough MIS was performed presumably to minimize technical error and reduce complications. These encounters were defined as the “specialized cohort” in our analysis and included 59 of the 1,308 hospitals in the original cohort. Predictor variables were selected a priori. Covariates included basic patient demographics: age, gender, race, insurance payer (public vs. private), median household income, Charlson comorbidity index, treatment year, treatment modality, and hospital-level factors (teaching status and geographic region).
Outcome selection
The primary outcome was postoperative complications; these were identified by ICD-9-CM codes (Appendix 2) which most closely corresponded to the complications described by the National Surgical Quality Improvement Program (NSQIP). Rare complications (≤15), while included in the analysis, were excluded from data tables per AHRQ requirements. For secondary outcomes, we analyzed the prevalence of MIS techniques for individual procedures over time and modeled the predictors of receiving MIS, adjusting for covariates.
Statistical analysis
We used descriptive statistics to describe the demographics of each cohort. Wald chi-square test was used to compare discrete variables and ANOVA was used for continuous variables to take into account the stratum, clusters, and weights present in the data set.
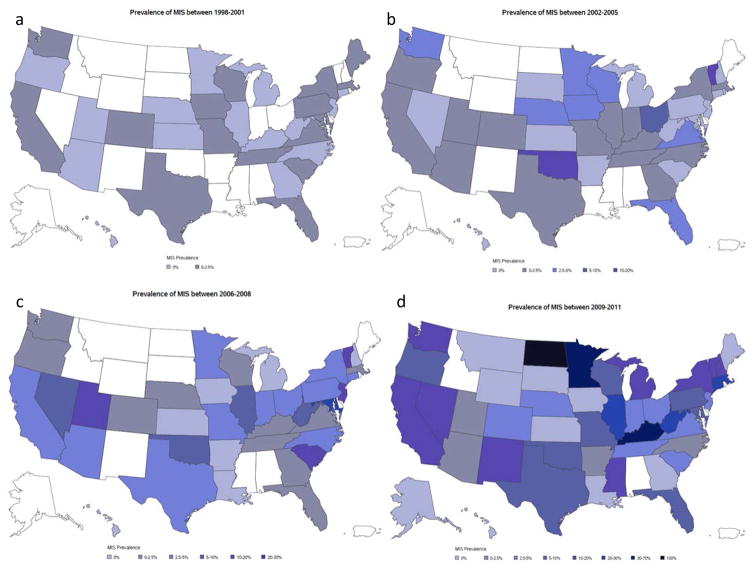
Using the “entire cohort,” we determined the overall frequency of complications and percentage of individual procedures performed using MIS. Encounters were subcategorized into four time periods (years 1998–2001, 2002–2005, 2006–2009, 2010–2011) to account for small numbers of observations in individual years. We then created heat maps (Fig. 1) to assess national trends in utilization (2012 was excluded from these maps as NIS did not report each hospital’s state for that year). States were excluded from the heat maps if they did not participate at any time in NIS.
Figure 1.
Heat maps showing national trends in utilization.
To ensure a fair comparison within the “specialized cohort,” we used propensity scores (PS) to match patients on surgery type. We adjusted for age, gender, race, Charlson Comorbidity Score, hospital bed size, hospital type, region, and year. Multiple imputation was used for missing data. We created 15 imputed data sets and exported them to R to perform propensity matching to create 15 matched data sets using the Matchit macro. As open surgery was expected to be significantly more prevalent than MIS, we used a control:case ratio of 3:1 using the “greedy” nearest neighbor method. We used PS matching jitter and love plots to assess how well the matching was performed. Matched data sets were exported to SAS and analyzed with PROC MIANALYZE. Generalized estimating equations were used to account for the complex survey design of NIS in addition to hospital clustering.
We modeled the 15 imputed, propensity-matched data sets to assess factors predictive of complications. We treated MIS vs. open as our primary predictor and adjusted for age, gender, race, Charlson Comorbidity Score, hospital bed size, hospital type/location, region, and year. Year was treated as a covariate in the model to take into account the varying rates of MIS by years that we observed in our figures. The same covariates were used to model the probability of receiving an MIS procedure.
A flowchart of this progression is provided in Appendix 3. An alpha of 0.05 and 95% confidence intervals (CI) were used as criteria for statistical significance. All analyses were performed using R version 3.1.0 and SAS 9.4 (SAS Institute, Cary, NC, USA).
Ethical approval
This protocol was reviewed by our institutional review board and deemed to be exempt.
Results
Demographics
We identified at total of 163,838 weighted encounters in the “overall cohort,” 70,273 of which were at centers performing more than five MIS procedures over the years studied (Table 1). Specialized centers had higher rates of teaching institutions (96 vs. 88% unspecialized, p<0.01), females (56.5% vs. 54% unspecialized, p<0.01), admissions in the South regions (42.5% vs. 27.6% unspecialized, p<0.01), lower rates of large bed sizes (56.4% vs. 60% unspecialized, p=0.01), lower complication rates (9% vs. 11.5% unspecialized, p<0.01), and, on average, treated younger children (mean age 4.8 ± 0.05 vs. 5.5 ± 0.06 years, p<0.0001). No significant variation in other demographic categories, including race (p=0.52) and insurance (p=0.08), was observed between specialized and unspecialized hospitals.
Table 1.
Comparison of patient characteristics at specialized and unspecialized hospitals
| Characteristic | Total (n=163,838) | Specialized (n=70,273) | Unspecialized (n=93,565) | p value |
|---|---|---|---|---|
| Age in years (SE) | 5.2 (0.05) | 4.8 (0.05) | 5.5 (0.06) | <0.0001a |
| Gender | <0.01b | |||
| Female | 89,693 (55.0%) | 39,609 (56.5%) | 50,084 (53.9%) | |
| Race | 0.52b | |||
| White | 86,015 (67.3%) | 39,980 (67.5%) | 46,035 (67.3%) | |
| Black | 8,854 (6.9%) | 4,223 (7.1%) | 4,631 (6.8%) | |
| Hispanic | 21,993 (17.2%) | 9,619 (16.2%) | 12,373 (18.1%) | |
| Other | 10,856 (8.5%) | 5,451 (9.2%) | 5,404 (7.9%) | |
| Insurance | 0.08b | |||
| Public | 51,510 (31.5%) | 23,840 (34.0%) | 27,671 (29.6%) | |
| Private | 101,289 (62.0%) | 41,772 (59.6%) | 59,517 (63.7%) | |
| Others | 10,642 (6.5%) | 4,468 (6.4%) | 6,174 (6.6%) | |
| ZIP code median income | 0.29b | |||
| Q1 | 24,936 (15.6%) | 11,358 (16.5%) | 13,578 (14.9%) | |
| Q2 | 37,911 (23.7%) | 16,733 (24.4%) | 21,178 (23.2%) | |
| Q3 | 42,565 (26.6%) | 17,751 (25.9%) | 24,814 (27.2%) | |
| Q4 | 54,458 (34.1%) | 22,807 (33.2%) | 31,651 (34.7%) | |
| Charlson Comorbidity Index | 0.12b | |||
| 0 | 139,609 (85.2%) | 60,232 (85.7%) | 79,377 (84.8%) | |
| 1 | 5,674 (3.5%) | 2,359 (3.4%) | 3,315 (3.5%) | |
| 2 | 14,088 (8.6%) | 5,980 (8.5%) | 8,108 (8.7%) | |
| ≥3 | 4,466 (2.7%) | 1,702 (2.4%) | 2,764 (3.0%) | |
| Hospital region | <0.01b | |||
| Northeast | 33,104 (20.2%) | 15,147 (21.6%) | 17,957 (19.2%) | |
| Midwest | 37,347 (22.8%) | 14,848 (21.1%) | 22,499 (24.0%) | |
| South | 55,706 (34.0%) | 29,848 (42.5%) | 25,858 (27.6%) | |
| West | 37,681 (23.0%) | 10,430 (14.8%) | 27,251 (29.1%) | |
| Teaching status | <0.01b | |||
| Rural | 3,598 (2.2%) | 982 (1.4%) | 2,616 (2.8%) | |
| Urban-nonteaching | 15,660 (9.6%) | 1,916 (2.7%) | 13,744 (14.7%) | |
| Urban-teaching | 144,106 (88.2%) | 67,143 (95.9%) | 76,963 (82.5%) | |
| Hospital bed size | 0.01b | |||
| Small | 27,132 (16.6%) | 7,789 (11.1%) | 19,343 (20.7%) | |
| Medium | 40,728 (24.9%) | 22,751 (32.5%) | 17,978 (19.3%) | |
| Large | 95,503 (58.5%) | 39,502 (56.4%) | 56,002 (60.0%) | |
| Probability of at least one complication | 17,042 (10.4%) | 6,307 (9.0%) | 10,734 (11.5%) | <0.01b |
| Surgery performed | ||||
| Ureteroneocystosto my | 83,641 (51.1%) | 37,625 (53.5%) | 46,016 (49.2%) | <0.01b |
| Ureteroureterostomy | 2,119 (1.3%) | 954 (1.4%) | 1,164 (1.2%) | 0.52b |
| Pyeloplasty | 38,229 (23.3%) | 15,375 (21.9%) | 22,854 (24.4%) | <0.001b |
| Nephrectomy | 28,616 (17.5%) | 11,491 (16.4%) | 17,126 (18.3%) | 0.02b |
| Partial/heminephrectomy | 6,424 (3.9%) | 2,624 (3.7%) | 3,800 (4.1%) | 0.29b |
| Appendicovesicostomy | 3,376 (2.1%) | 1,443 (2.1%) | 1,933 (2.1%) | 0.97b |
| Enterocystoplasty | 7,703 (4.7%) | 3,615 (5.1%) | 4,088 (4.4%) | 0.07b |
| Bladder neck sling | 988 (0.6%) | 505 (0.7%) | 483 (0.5%) | 0.08b |
Weighted ANOVA.
Wald chi-square.
Following exclusion of centers performing fewer than five MIS procedures, the “specialized cohort” consisted of 66,510 open and 3,763 MIS surgeries (Table 2). In this cohort, patients receiving MIS were, on average, older (7.8 ± 0.23 vs. 4.7 ± 0.05, p<0.0001), had higher rates of males (51.6% vs. 43%, p<0.0001), and lower rates of comorbidity scores ≥2 (10.6% vs. 11.2%; p<0.001).
Table 2.
Demographics for specialized hospital cohort, according to procedure type
| Characteristics | Total (n=70,273) | MIS (n=3,763) | Open (n=66,510) | p value |
|---|---|---|---|---|
| Age in years (SE) | 4.8 (0.05) | 7.8 (0.23) | 4.7 (0.05) | <0.0001a |
| Gender | <0.0001b | |||
| Female | 39,609 (56.5%) | 1,808 (48.4%) | 37,801 (57.0%) | |
| Race | 0.10b | |||
| White | 39,980 (67.5%) | 1,861 (57.8%) | 38,119 (68.0%) | |
| Black | 4,223 (7.1%) | 258 (8.0%) | 3,964 (7.1%) | |
| Hispanic | 9,619 (16.2%) | 685 (21.3%) | 8,934 (15.9%) | |
| Other | 5,451 (9.2%) | 413 (12.8%) | 5,038 (9.0%) | |
| Insurance | <0.0001b | |||
| Public | 23,840 (34.0%) | 1,513 (40.3%) | 22,326 (33.7%) | |
| Private | 41,772 (59.6%) | 2,093 (55.8%) | 39,679 (59.8%) | |
| Others | 4,468 (6.4%) | 147 (3.9%) | 4,321 (6.5%) | |
| Income | 0.02b | |||
| Q1 | 11,358 (16.5%) | 709 (19.2%) | 10,649 (16.4%) | |
| Q2 | 16,733 (24.4%) | 879 (23.8%) | 15,854 (24.4%) | |
| Q3 | 17,751 (25.9%) | 1,058 (28.7%) | 16,693 (25.7%) | |
| Q4 | 22,807 (33.2%) | 1,046 (28.3%) | 21,761 (33.5%) | |
| Charlson Comorbidity Index | <0.001b | |||
| 0 | 60,232 (85.7%) | 3,299 (87.7%) | 56,934 (85.6%) | |
| 1 | 2,359 (3.4%) | 198 (5.3%) | 2,161 (3.2%) | |
| 2 | 5,980 (8.5%) | 198 (5.3%) | 5,782 (8.7%) | |
| ≥3 | 1,702 (2.4%) | 68 (1.8%) | 1,634 (2.5%) | |
| Hospital region | <0.01b | |||
| Northeast | 15,147 (21.6%) | 782 (20.8%) | 14,365 (21.6%) | |
| Midwest | 14,848 (21.1%) | 857 (22.8%) | 13,992 (21.0%) | |
| South | 29,848 (42.5%) | 1,055 (28.0%) | 28,793 (43.3%) | |
| West | 10,430 (14.8%) | 1,069 (28.4%) | 9,361 (14.1%) | |
| Teaching status | <0.0001b | |||
| Rural | 982 (1.4%) | 33 (0.9%) | 950 (1.4%) | |
| Urban-nonteaching | 1,916 (2.7%) | 147 (3.9%) | 1,769 (2.7%) | |
| Urban-teaching | 67,143 (95.9%) | 3,540 (95.2%) | 63,604 (95.9%) | |
| Hospital bed size | <0.0001b | |||
| Small | 7,789 (11.1%) | 166 (4.5%) | 7,624 (11.5%) | |
| Medium | 22,751 (32.5%) | 1,363 (36.6%) | 21,388 (32.2%) | |
| Large | 39,502 (56.4%) | 2,191 (58.9%) | 37,311 (56.3%) | |
| Probability of at least one complication | 6,307 (9.0%) | 265 (7.0%) | 6,042 (9.1%) | <0.01b |
| Surgery performed | ||||
| Ureteroneocystostomy | 37,625 (53.5%) | 687 (18.2%) | 36,938 (55.5%) | <0.0001b |
| Ureteroureterostomy | 954 (1.4%) | 56 (1.5%) | 898 (1.4%) | 0.81b |
| Pyeloplasty | 15,375 (21.9%) | 1,599 (42.5%) | 13,776 (20.7%) | <0.0001b |
| Nephrectomy | 11,491 (16.4%) | 1,131 (30.0%) | 10,360 (15.6%) | <0.0001b |
| Partial/heminephrectomy | 2,624 (3.7%) | 268 (7.1%) | 2,356 (3.5%) | <0.01b |
| Appendicovesicostomy | 1,443 (2.1%) | 59 (1.6%) | 1,385 (2.1%) | 0.25b |
| Enterocystoplasty | 3,615 (5.1%) | 38 (1.0%) | 3,577 (5.4%) | <0.0001b |
| Sling | 505 (0.7%) | * | 490 (0.7%) | 0.03b |
Weighted ANOVA.
Wald chi-square.
Use of minimally invasive surgery
Use of MIS techniques increased significantly over time for several procedures, most prominently for nephrectomy (Summary Figure). Adjusting for other covariates, hospital setting had a statistically significant association to surgery type, with patients seen at rural hospitals having lower odds of receiving an MIS procedure compared with those seen at urban teaching hospitals (OR 0.43, p=0.39). By contrast, urban nonteaching hospitals had higher odds compared with urban teaching to perform an MIS procedure (OR 2.44, p=0.02). Heat maps displaying national geographic trends in uptake of MIS are shown in Figure 1.
Postoperative complications
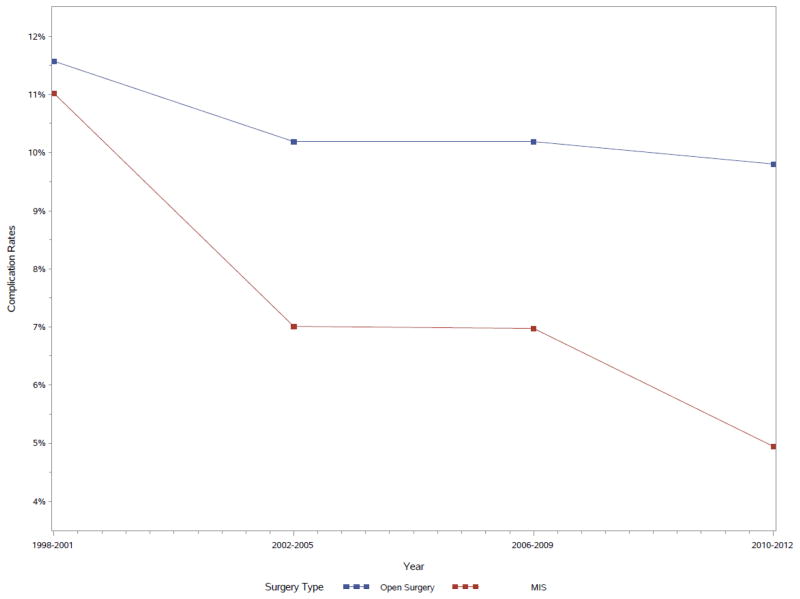
The overall rate of complications was lower in patients undergoing MIS compared with open surgery (6.3% vs. 10.6%, p<0.0001). Specialized centers had a significantly lower overall rate of complications than unspecialized centers (9% vs. 11.5%, p<0.01). Within specialized centers, MIS had lower complication rates than open procedures (7% vs. 9.1%, p<0.01); this finding was consistent even after adjusting for other factors (OR 0.70, p=0.03, Table 3). Complication frequency – initially similar at the start of the study period – decreased more steeply for MIS procedures over time than for open procedures (Fig. 2).
Table 3.
Multivariable model predicting the odds of postoperative complications
| Variables | OR (95% CI) | p value |
|---|---|---|
| Surgery type | ||
| Open surgery | Reference | |
| MIS | 0.70 (0.51, 0.96) | 0.03 |
| Age | 1.05 (1.02, 1.08) | <0.01 |
| Gender | ||
| Male | Reference | |
| Female | 1.43 (1.07, 1.90) | 0.01 |
| Race | ||
| Other | Reference | |
| Caucasian | 0.66 (0.48, 0.93) | 0.02 |
| CCS | 1.79 (1.48, 2.15) | <0.01 |
| Bed size | ||
| Large bed | Reference | |
| Small bed | 1.02 (0.57, 1.85) | 0.94 |
| Medium bed | 0.57 (0.39, 0.84) | <0.01 |
| Hospital location and type | ||
| Urban teaching | Reference | |
| Rural | 0.72 (0.12, 4.42) | 0.72 |
| Urban nonteaching | 0.30 (0.09, 1.06) | 0.06 |
| Hospital region | ||
| West | Reference | |
| Northeast | 0.72 (0.42, 1.23) | 0.23 |
| Midwest | 0.76 (0.47, 1.22) | 0.26 |
| South | 0.71 (0.47, 1.09) | 0.12 |
| Treatment year | ||
| 1998–2001 | Reference | |
| 2002–2005 | 1.17 (0.25, 5.53) | 0.84 |
| 2006–2009 | 1.18 (0.25, 5.46) | 0.83 |
| 2010–2012 | 1.19 (0.27, 5.29) | 0.82 |
Correlation of hospitals and year of admission used.
Figure 2.
Complication rates of open surgery and MIS by year.
Additionally, for each point increase on the Charlson Comorbidity Index, patients had significantly higher odds of experiencing a complication (OR 1.79, p<0.01). Specific complications are detailed in Table 4.
Table 4.
Complications according to surgical technique, entire cohort.
| Complication | Open (%) (n = 163,447) | MIS (%) (n = 5,761) | p Value |
|---|---|---|---|
| Surgical site infection (deep and superficial) | 141 (0.1%) | a | 0.51 |
| Peritoneal abscess | 78 (0%) | a | — |
| Acute renal failure | 1,813 (1.1%) | 31 (0.5%) | 0.01 |
| Urinary tract infection | 9,027 (5.5%) | 192 (3.3%) | <0.001 |
| Urinary complications | 2,627(1.6%) | 47 (0.8%) | 0.01 |
| Respiratory complications | 2,156(1.3%) | 21 (0.4%) | <0.001 |
| Pneumonia | 814 (0.5%) | a | 0.0001 |
| Acute respiratory distress syndrome | 130 (0.1%) | a | 0.95 |
| Acute respiratory failure | 587 (0.4%) | a | 0.01 |
| Postoperative respiratory insufficiency | 798 (0.5%) | a | 0.02 |
| Systemic sepsis | 920 (0.6%) | 16 (0.3%) | 0.11 |
| Myocardial infarction | a | a | — |
| Deep vein thrombosis | 45 (0%) | a | — |
| Cardiac complications | 315 (0.2%) | 15 (0.3%) | 0.68 |
| Cardiac arrest | 170 (0.1%) | a | — |
| Pulmonary embolism | a | a | — |
| Bleeding | 1,859 (1.1%) | 62(1.1%) | 0.8 |
| Renal complications | 841 (0.5%) | 132 (2.3%) | <0.001 |
| Ureteral complications | 399 (0.2%) | 40 (0.7%) | 0.06 |
Denotes <15 occurrences. Not reported per AHRQ Data Use Agreement.
Relative to patients undergoing open procedures, MIS patients had significantly lower rates of acute renal failure (p=0.01), urinary complications (p<0.01), and respiratory complications (p<0.0001). However, a relatively higher proportion of MIS patients experienced general postoperative renal complications relative to open patients (p<0.0001).
Discussion
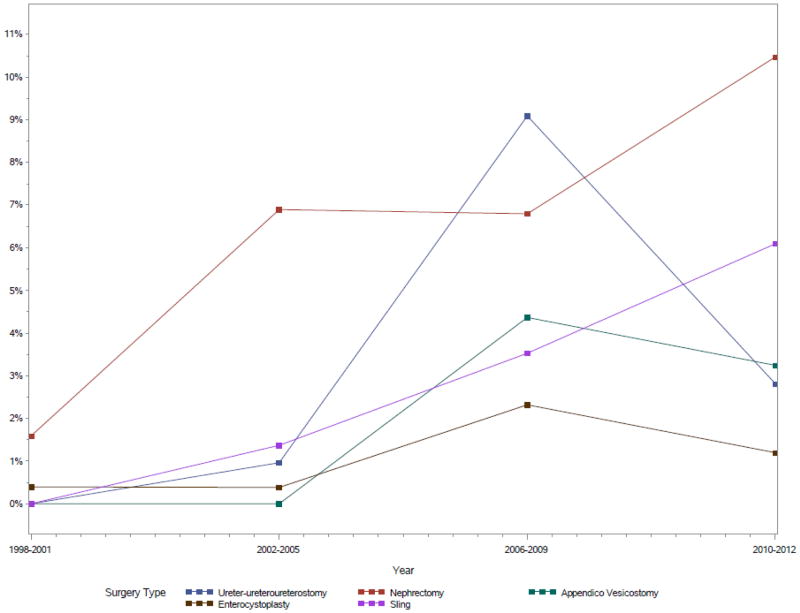
Our analysis of 70,273 weighted encounters revealed that MIS use was associated with a significantly lower incidence of postoperative complications among children undergoing urinary tract procedures. Adjusting for age, gender, comorbidity score, and hospital-level clustering effects, children who underwent MIS procedures had a 30% reduction in their odds of developing in-hospital NSQIP-defined complications relative to those undergoing open procedures, contrary to our expectations. Children who underwent MIS at specialized hospitals were more likely to be older, male, and had slightly higher rates of private insurance than those treated with open surgery. These data further confirm what has previously been known anecdotally: that MIS use has increased significantly over the past two decades for some common pediatric urologic procedures nationwide. Rates of MIS nephrectomies, ureteroureterostomies, appendicovesicostomies, and bladder neck slings in particular have more than doubled since the year 2000, and by 2011 MIS cases accounted for 7% of the eight pediatric urologic procedures analyzed.
As noted by Lorenzo et al., the concept of MIS can be tantalizing given the potential for comparable-or-better outcomes relative to open approaches with less tissue trauma and greater potential patient satisfaction [22]. MIS has been associated with comparable-or-better lengths of stay (LOS), recovery times, and postoperative morbidity and mortality in prior literature from adult and pediatric urologic case-series, and other surgical subspecialties [7–9,14,23]. Indeed, LOS and recovery time in particular may factor prominently in our observed incidence of pulmonary complications, given known risks for atelectasis, pneumonia, and upper-respiratory tract infections associated with prolonged hospitalization [24]. Better visualization within the peritoneum and retroperitoneum theoretically could permit more accurate dissection which, in turn, may further reduced procedure-related morbidity [25].
While similar at the outset of the study period, annual MIS complication rates declined more rapidly than those for open procedures, which remained mostly stable over time. Although undoubtedly, there are several potential reasons for this observation including technological improvements over the past two decades, we believe it most likely attributable to known “learning-curve” effects associated with MIS as described by Georgeson and others [1,13,26,27]. Such effects often follow the advent and dissemination of new medical technologies, as familiarity and precision increase with greater experience [27]. Early exposure and continued education geared toward operator improvement can greatly reduce the “steepness” of this curve, and adequately trained surgeons are consequently more likely to consider MIS approaches for their patients [28,29]. We note, however, that several authors have reported considerable dissatisfaction among American urological residents surveyed about MIS training during residency, with 60–85% reporting subjectively inadequate laparoscopic and robotic exposure [30,31]. Further discussion is warranted on how best to improve MIS training in graduate medical education.
Demographic differences noted between our MIS and open patient cohorts suggest a strong degree of modality-specific patient selection, particularly vis-à-vis age and comorbidities. Older children may be felt to be better candidates for MIS given larger and better-developed abdominopelvic spaces, which afford greater maneuverability of laparoscopic instruments and less risk of accidental injury. Such children are also more stable physiologically, and likely more tolerant of operative and anesthetic insults in the event of intraoperative open conversion should MIS fail. This may similarly explain why children with higher CCS scores were slightly more likely to undergo open surgery, rather than MIS.
The findings of our study must be viewed in the context of its design limitations. NIS represents a 20% stratified sample of US hospital admissions. Consequently, results derived from it may not be generalizable to encounters not in the sample pool. Non-participation by specific centers with high pediatric surgical volumes may similarly reduce the accuracy of our state-level reporting of MIS prevalence over time. We note, however, that NIS provides meticulous tracking of discharge and hospital weights to minimize sampling bias risk. Because NIS represents admission-based rather than patient-based data, it is impossible to track a given patient across time. We were unable to assess longer-term outcomes nor whether individual patients had multiple admissions. As a large, retrospective, administrative database, NIS may be affected by miscoding bias – rendering our analysis sensitive to the accuracy of procedure coding in NIS. Although the accuracy level of NIS is high for an administrative database, it is possible that at least some portion of our cohort may be incorrectly coded. This is particularly true for our method to identify MIS cases, which relies on a concurrent ICD-9 code being present. While this same definition has been successfully used in several other reports [18–21], it is likely that this method resulted in some MIS cases being incorrectly labeled as “open.” We would anticipate that miscoding bias would, in this case, have biased towards the null; in other words, systematically mistaking MIS cases for open should tend to make the two cohorts more similar. Thus, while miscoding bias is very likely to have been present in our results, we would not anticipate that miscoding bias alone would invalidate our results.
Similarly, the NSQIP complications we identified may represent associated comorbidities and not true postoperative complications, as NIS does not provide temporal relationships between different diagnosis codes. Despite these limitations, we note that the NIS database is rigorously monitored and audited for coding accuracy and, therefore, represents a reasonably reliable panorama of the characteristics of an inpatient surgical cohort.
Selection bias is likely an issue in these data. We suspect that patient selection and learning curve issues are factors that play a role in procedure choice; however, these data unfortunately cannot prove this hypothesis. In addition, the lack of accurate, contemporary ambulatory surgery datasets in pediatrics left us unable to study encounters not associated with inpatient admissions – notably patients who undergo procedures in ambulatory surgery centers, as many children undoubtedly do. Although the number of such patients not included in our cohort is believed to be relatively small, the possibility remains that such data may alter our understanding of patient and center-specific characteristics reported in this study.
However, it is important to note that the choice of operative modality is, undoubtedly, multifactorial and patient/setting-specific. Either MIS or open approaches may be more appropriate for a particular child based on anatomic/physiologic considerations, institutional resources, provider proficiency, parental preferences, and procedural costs. Further investigations more closely examining these factors as they pertain to modality choice are warranted.
Conclusions
There is steadily increasing use of MIS for pediatric urology procedures, although rates of MIS use vary widely between procedures. MIS was associated with a lower NSQIP-defined postoperative complication rate. Higher-volume MIS centers have a lower complication rate than lower-volume centers.
Supplementary Material
Multivariable model for odds of receiving MIS over open surgery
Figure.
Prevalence of MIS by procedure and year (p<0.03 for all).
Appendix 1. Urologic procedure codes for study inclusion
| Procedure | ICD-9 procedure code |
|---|---|
| Ureteral reimplantation | 56.74, 59.3 |
| Ureteroureterostomy | 56.75 |
| Pyeloplasty | 55.86, 55.87 |
| Nephrectomy | 55.5 |
| Partial nephrectomy | 55.4 |
| Appendicovesicostomy | 57.88 |
| Enterocystoplasty | 57.87 |
| Bladder outlet repair | 59.4, 59.5, 59.6 |
Appendix 2. ICD-9 codes used to identify complications
| Complications | ICD-9 codes |
|---|---|
| Surgical site infection (superficial) | 998.32 |
| Surgical site infection (deep) | 998.31 |
| Peritoneal abscess | 567.22 |
| Urinary tract infection | 599 |
| Urinary complications | 997.5 |
| Acute renal failure | 584.x, 586.x |
| Respiratory complications | 997.3 |
| Pneumonia | 481–487, 507 |
| Post-operative respiratory insufficiency | 518.5 |
| Acute respiratory distress syndrome | 518.82 |
| Systemic sepsis | 790.7, 038.x |
| Pulmonary emboli | 415.1, 415.11, 415.19 |
| Mechanical ventilation >96 hours | 96.72 |
| Cerebrovascular accident | 997.02 |
| Cardiac complications | 997.1 |
| Myocardial infarction | 410.x |
| Cardiac arrest | 427.5 |
| Bleeding | 285.1, 998.11 |
| Deep vein thrombosis | 453.4, 453.40, 453.9 |
Appendix 3. Complications according to surgical technique, entire cohort
| Complication | Open (%) (n = 158,246) | MIS (%) (n = 5,593) | Total (%) (n = 163,839) | p value |
|---|---|---|---|---|
| SSI (deep and superficial) | 135 (0.1%) | a | 145 (0.1%) | 0.49 |
| Peritoneal abscess | 75 (0.05%) | a | 75 (0.05%) | 1.00 |
| Acute renal failure | 1753 (1.1%) | 30 (0.5%) | 1782 (1.1%) | 0.01 |
| UTI | 8,758 (5.5%) | 186 (3.3%) | 8,944 (5.5%) | <0.0001 |
| Urinary complications | 2,536 (1.6%) | 45 (0.8%) | 2,581 (1.6%) | <0.01 |
| Respiratory complications | 2,090 (1.3%) | 20 (0.4%) | 2,111 (1.3%) | <0.0001 |
| Pneumonia | 788 (0.5%) | a | 793 (0.5%) | <0.0001 |
| ARDS | 125 (0.1%) | a | 130 (0.1%) | 0.94 |
| Acute respiratory failure | 568 (0.4%) | a | 573 (0.3%) | 0.01 |
| Postoperative respiratory insufficiency | 768 (0.5%) | a | 777 (0.5%) | 0.01 |
| Systemic sepsis | 889 (0.6%) | 15 (0.3%) | 904 (0.6%) | 0.10 |
| MI | a | a | a | 1.00 |
| DVT | 44 (0.03%) | a | 44 (0.03%) | 1.00 |
| Cardiac complications | 303 (0.2%) | a | 317 (0.2%) | 0.70 |
| Cardiac arrest | 164 (0.1%) | a | 164 (0.1%) | 0.63 |
| Pulmonary embolism | a | a | a | 1.00 |
| Bleeding | 1801 (1.1%) | 59 (1.1%) | 1860 (1.1%) | 0.77 |
| Renal complications | 814 (0.5%) | 128 (2.3%) | 942 (0.6%) | <0.0001 |
| Ureteral complications | 385 (0.2%) | 39 (0.7%) | 424 (0.3%) | 0.06 |
Denotes <15 occurrences. Not reported per AHRQ Data Use Agreement.
Footnotes
Conflict of interest
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Georgeson KE, Owings E. Advances in minimally invasive surgery in children. Am J Surg. 2000;180:362–4. doi: 10.1016/s0002-9610(00)00554-7. [DOI] [PubMed] [Google Scholar]
- 2.Albersen M, Cartwright R, Choyke P, Goldenberg SL, Goldman H, Lawrentschuk N, et al. Looking forward, looking back-10 years in urology. Nat Rev Urol. 2014;11:649–55. doi: 10.1038/nrurol.2014.263. [DOI] [PubMed] [Google Scholar]
- 3.Firilas AM, Jackson RJ, Smith SD. Minimally invasive surgery: the pediatric surgery experience. J Am Coll Surg. 1998;186:542–4. doi: 10.1016/s1072-7515(98)00087-8. [DOI] [PubMed] [Google Scholar]
- 4.Traxel EJ, Minevich EA, Noh PH. A review: the application of minimally invasive surgery to pediatric urology: upper urinary tract procedures. Urology. 2010;76:122–33. doi: 10.1016/j.urology.2009.11.072. [DOI] [PubMed] [Google Scholar]
- 5.Malkan AD, Loh AHP, Sandoval JA. Minimally invasive surgery in the management of abdominal tumors in children. J Pediatr Surg. 2014;49:1171–6. doi: 10.1016/j.jpedsurg.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 6.Romao RLP, Weber B, Gerstle JT, Grant R, Pippi Salle JL, Bägli DJ, et al. Comparison between laparoscopic and open radical nephrectomy for the treatment of primary renal tumors in children: Single-center experience over a 5-year period. J Pediatr Urol. 2014;10:488–94. doi: 10.1016/j.jpurol.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Smith RP, Oliver JL, Peters CA. Pediatric robotic extravesical ureteral reimplantation: comparison with open surgery. J Urol. 2011;185:1876–81. doi: 10.1016/j.juro.2010.12.072. [DOI] [PubMed] [Google Scholar]
- 8.Barbosa JA, Barayan G, Gridley CM, Sanchez DC, Passerotti CC, Houck CS, et al. Parent and patient perceptions of robotic vs open urological surgery scars in children. J Urol. 2013;190:244–50. doi: 10.1016/j.juro.2012.12.060. [DOI] [PubMed] [Google Scholar]
- 9.Colodny AH. Laparoscopy in pediatric urology: too much of a good thing? Semin Pediatr Surg. 1996;5:23–9. [PubMed] [Google Scholar]
- 10.Passerotti CC, Nguyen HT, Retik AB, Peters CA. Patterns and predictors of laparoscopic complications in pediatric urology: the role of ongoing surgical volume and access techniques. J Urol. 2008;180:681–5. doi: 10.1016/j.juro.2008.04.042. [DOI] [PubMed] [Google Scholar]
- 11.Lawrence K, McWhinnie D, Goodwin A, Gray A, Gordon J, Storie J, et al. An economic evaluation of laparoscopic versus open inguinal hernia repair. J Public Health Med. 1996;18:41–8. doi: 10.1093/oxfordjournals.pubmed.a024460. [DOI] [PubMed] [Google Scholar]
- 12.Waldhausen JH, Tapper D. Is pediatric laparoscopic splenectomy safe and cost-effective? Arch Surg. 1997;132:822–4. doi: 10.1001/archsurg.1997.01430320024003. [DOI] [PubMed] [Google Scholar]
- 13.Esposito C, Lima M, Mattioli G, Mastroianni L, Centonze A, Monguzzi GL, et al. Complications of pediatric urological laparoscopy: mistakes and risks. J Urol. 2003;169:1490–2. doi: 10.1097/01.ju.0000055256.43528.f6. discussion 2. [DOI] [PubMed] [Google Scholar]
- 14.Elsamra S, Theckumparampil N, Garden B, Alom M, Waingankar N, Leavitt DA, et al. Open, Laparoscopic, and Robotic Ureteroneocystotomy for Benign and Malignant Ureteral Lesions: A Comparison of over 100 Minimally Invasive Cases. J Endourol. 2014;28(12):1455–9. doi: 10.1089/end.2014.0243. [DOI] [PubMed] [Google Scholar]
- 15.Tobias JD. Anaesthesia for minimally invasive surgery in children. Best Pract Res Clin Anaesthesiol. 2002;16:115–30. doi: 10.1053/bean.2001.0211. [DOI] [PubMed] [Google Scholar]
- 16.Tejwani R, Wang HS, Young BJ, Greene NH, Wolf S, Wiener JS, et al. Increased pediatric sub-specialization is associated with decreased surgical complication rates for inpatient pediatric urology procedures. J Pediatr Urol. 2016;12:388e1–e7. doi: 10.1016/j.jpurol.2016.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang HH, Tejwani R, Zhang H, Wiener JS, Routh JC. Hospital Surgical Volume and Associated Postoperative Complications of Pediatric Urological Surgery in the United States. J Urol. 2015;194:506–11. doi: 10.1016/j.juro.2015.01.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghani KR, Sukumar S, Sammon JD, Rogers CG, Trinh QD, Menon M. Practice patterns and outcomes of open and minimally invasive partial nephrectomy since the introduction of robotic partial nephrectomy: results from the nationwide inpatient sample. J Urol. 2014;191:907–12. doi: 10.1016/j.juro.2013.10.099. [DOI] [PubMed] [Google Scholar]
- 19.Kim SP, Shah ND, Karnes RJ, Weight CJ, Shippee ND, Han LC, et al. Hospitalization costs for radical prostatectomy attributable to robotic surgery. Eur Urol. 2013;64:11–6. doi: 10.1016/j.eururo.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 20.Parsons JK, Messer K, Palazzi K, Stroup SP, Chang D. Diffusion of surgical innovations, patient safety, and minimally invasive radical prostatectomy. JAMA Surg. 2014;149:845–51. doi: 10.1001/jamasurg.2014.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parsons JK, Rangarajan SS, Palazzi K, Chang D. A National, Comparative Analysis of Perioperative Outcomes of Open and Minimally Invasive Simple Prostatectomy. J Endourol. 2015;29:919–24. doi: 10.1089/end.2014.0879. [DOI] [PubMed] [Google Scholar]
- 22.Lorenzo AJ, Romao RL. The Evolving Role of Minimally Invasive Surgery in Pediatric and Adolescent Urologic Oncology. Urology. 2016;91:180–9. doi: 10.1016/j.urology.2015.12.023. [DOI] [PubMed] [Google Scholar]
- 23.McCullough TC, Barret E, Cathelineau X, Rozet F, Galiano M, Vallancien G. Role of robotics for prostate cancer. Curr Opin Urol. 2009;19:65–8. doi: 10.1097/MOU.0b013e32831aedcf. [DOI] [PubMed] [Google Scholar]
- 24.Brooks-Brunn JA. Predictors of postoperative pulmonary complications following abdominal surgery. Chest. 1997;111:564–71. doi: 10.1378/chest.111.3.564. [DOI] [PubMed] [Google Scholar]
- 25.Camarillo DB, Krummel TM, Salisbury JK., Jr Robotic technology in surgery: past, present, and future. Am J Surg. 2004;188:2S–15S. doi: 10.1016/j.amjsurg.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 26.Mabrouk M, Frumovitz M, Greer M, Sharma S, Schmeler KM, Soliman PT, et al. Trends in laparoscopic and robotic surgery among gynecologic oncologists: A survey update. Gynecol Oncol. 2009;112:501–5. doi: 10.1016/j.ygyno.2008.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.See WA, Cooper CS, Fisher RJ. Predictors of laparoscopic complications after formal training in laparoscopic surgery. JAMA. 1993;270:2689–92. [PubMed] [Google Scholar]
- 28.Shalhav AL, Dabagia MD, Wagner TT, Koch MO, Lingeman JE. Training postgraduate urologists in laparoscopic surgery: the current challenge. J Urol. 2002;167:2135–7. [PubMed] [Google Scholar]
- 29.Shay BF, Thomas R, Monga M. Urology practice patterns after residency training in laparoscopy. J Endourol. 2002;16:251–6. doi: 10.1089/089277902753752232. [DOI] [PubMed] [Google Scholar]
- 30.Duchene DA, Moinzadeh A, Gill IS, Clayman RV, Winfield HN. Survey of residency training in laparoscopic and robotic surgery. J Urol. 2006;176:2158–66. doi: 10.1016/j.juro.2006.07.035. discussion 67. [DOI] [PubMed] [Google Scholar]
- 31.Wang DS, Winfield HN. Survey of urological laparoscopic practice patterns in the midwest. J Urol. 2004;172:2282–6. doi: 10.1097/01.ju.0000145384.99454.be. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Multivariable model for odds of receiving MIS over open surgery