Figure 2.
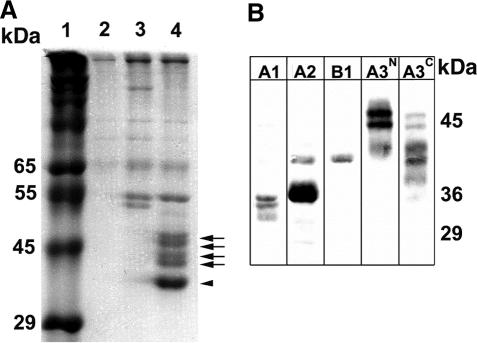
hnRNPs A1, A2, A3 and their isoforms are isolated in pull downs with a telomeric oligonucleotide. (A) Rat brain protein extract was incubated with superparamagnetic particles bearing no oligonucleotide (track 2), the scrambled A2RE ribonucleotide NS1 (track 3) or d(TTAGGG)4 (track 4). Bound proteins were eluted using the SDS–gel electrophoresis sample preparation solution. Proteins were separated on a 15 cm 12% SDS–polyacrylamide gel and stained with Coomassie Blue R250. The four arrows on the right indicate the hnRNP A3 isoforms (47) and the arrowhead indicates hnRNP A2. The masses of standard proteins (track 1) are shown in kDa (the standards used in this experiment suggest that the slowest migrating hnRNP A3 band has a molecular mass over 45 kDa; however, it normally migrates with an apparent mass of 42 kDa). (B) Proteins isolated as above were separated by SDS–PAGE and electroblotted onto a polyvinylidine difluoride membrane. Antibodies directed against peptides in hnRNPs A1, A2/B1(labeled A2), B1, and the two higher molecular weight isoforms (A3N) and all four isoforms of hnRNP A3 (A3C) were used to demonstrate the presence of these proteins in the eluate (47). Molecular masses, in kDa, are indicated on the right.