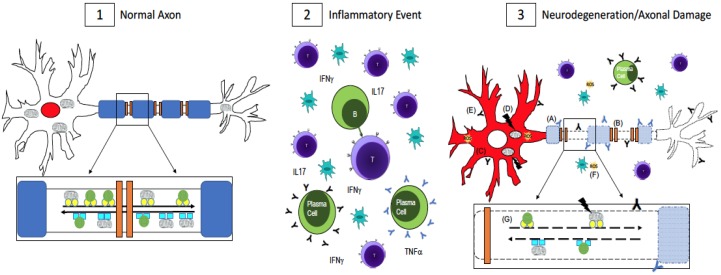
Figure 1.
Axonal Damage in multiple sclerosis (MS). (1) In a normal, healthy axon, myelin (blue) wraps around the axon and ion channels (orange) are clustered in the unmyelinated nodes of Ranvier. This enables saltatory conduction for fast signal transmission down the axon. RNA binding proteins, which maintain RNA homeostasis, are localized to the nucleus (red). Mitochondria and other cargo (green circles) are transported retrogradely along the axon by dynein (turquoise squares, inset) while anterograde transport is done by kinesin motor proteins (yellow circles, inset). Transport is fast and uninterrupted because the axon is undamaged, has no energy shortage, and the motor proteins are intact; (2) In MS, there is central nervous system infiltration of T-cells, B-cells, plasma cells, and macrophages, which lead to a cascade of events including the release of pro-inflammatory cytokines and antibodies, which are thought to be harmful to both myelin and neurons and axons. [43,44,45,46,47,48,49]; (3) Axonal damage and neurodegeneration occur simultaneously with inflammation. There is ongoing demyelination due to antibodies against myelin antigens (blue Y, A) [43,44,45]. Demyelination leads to the redistribution of ion channels (B), which impairs conduction along the axon [32,33]. The redistribution of RNA binding proteins from their normal nuclear location (panel 1, red) to the cytoplasm (panel 3, C, red) is a pathological feature of neuronal degeneration in neurological diseases [25,26,27,28,30,31]. Mutations in mitochondrial DNA (D) can impair the cell’s ability to generate enough ATP while antibodies to non-myelin antigens (black Y, E) damage axons [46,47,48,49]. Reactive oxygen species (yellow, F) could be released from activated microglia or as a result of dysfunctional mitochondria [37,38,39,40,41]. Impaired fast axonal transport (G) is also evident in MS [9,34,35,36]. A combination of these events, as opposed to one in particular, contributes to neurodegeneration and axonal damage in MS.