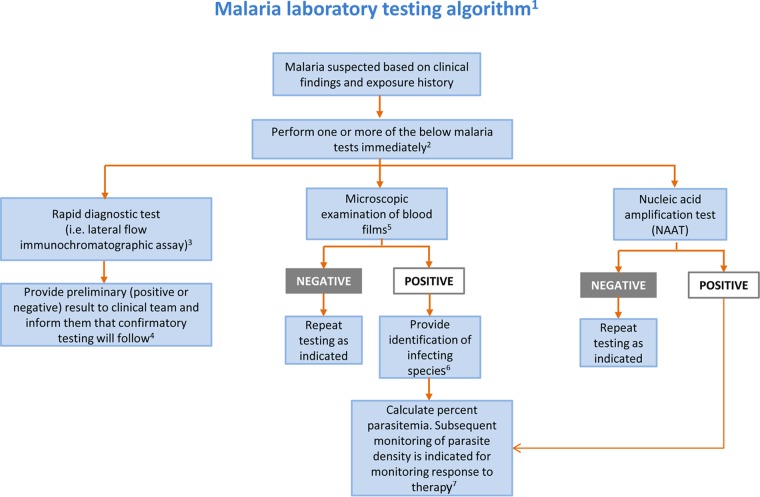
FIG 1.
Malaria laboratory testing algorithm. 1Algorithm includes only commonly used/available laboratory methods. Serology should not be used for detection of acute malaria. 2Malaria can be a rapidly fatal disease, particularly when due to P. falciparum, and less commonly P. vivax and P. knowlesi, and testing must be performed on a STAT basis. If testing is not available at the local laboratory, then arrangements must be made with another nearby laboratory that can provide immediate testing. A single negative test does not rule out malaria. Perform testing every 6 to 8 h for up to 3 days if clinically indicated. Other laboratory tests (e.g., complete blood count with differential, electrolyte panel, blood glucose, bilirubin, urinalysis, blood cultures) may be indicated to assess the severity of malaria and evaluate other potential causes of the patient's illness. 3Rapid screening tests such as lateral flow immunochromatographic assays generally provide sensitive detection of high levels of P. falciparum and P. vivax infection (i.e., 2,000 parasites/μl) but lack sufficient sensitivity for detecting low levels of parasitemia (i.e., ≤200 parasites/μl) and other Plasmodium species. 4Confirmatory testing may be performed by microscopic examination of blood films or NAAT. 5Examination of both thick and thin blood films is the gold standard for malaria diagnosis. 6If necessary, refer for confirmation of species identification by blood film microscopy or NAAT at a reference laboratory. 7Percent parasitemia is calculated using microscopic examination of thick or thin blood films. (Used with permission of Mayo Foundation for Medical Education and Research. All rights reserved.)