Fig. 2.
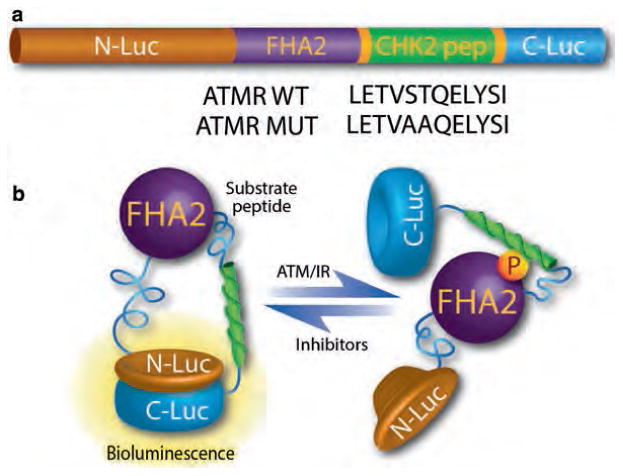
The components and functional basis of the ATM kinase activity reporter. (a) The ATM reporter consists of a phospho Ser/Thr-binding domain (FHA2), substrate peptide (CHK2), and split firefly luciferase. The substrate sequence is flanked by short linker sequences at either end. The functional basis of the reporter is demonstrated in part figure (b). In the presence of ATM kinase activity, the CHK2 target peptide is phosphorylated, resulting in interaction with the FHA2 domain, producing stearic constraints that inhibit functional reconstitution of the luciferase. In the absence of ATM kinase activity such as by small molecule inhibitors, siRNA-mediated knockdown (of ATM), or over-expression of phosphatases, the CHK2 consensus sequence is hypo-phosphorylated, allowing for lucif- erase enzyme reconstitution and increased bioluminescent activity
