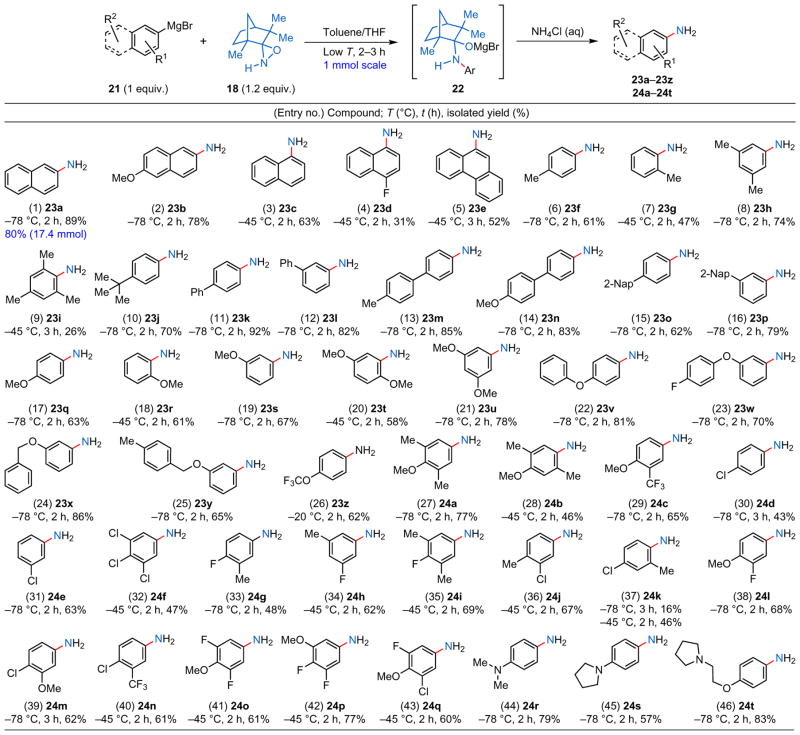
Table 1.
Direct primary amination of arylmagnesium halide substrates with N–H oxaziridine 18.
All the aromatic Grignard reagents (21) were prepared from the corresponding aryl halides using turnings of freshly activated Mg metal and THF as the solvent. The concentration of the arylmetal solution was targeted to be around 0.5 M, but was carefully determined by titration immediately before use. The amination reactions were conducted on a 1 mmol scale at the indicated temperature and considered complete on full consumption of the aminating agent (18) by thin-layer chromatography analysis; a number of experiments showed that 18 undergoes decomposition in the presence of strong metal bases.