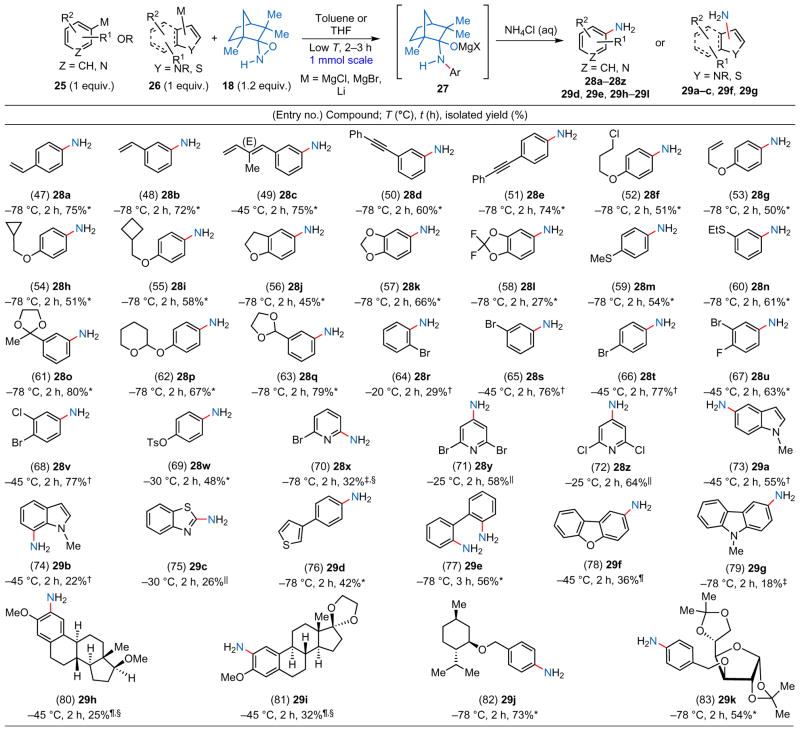
Table 2.
Direct primary amination of aromatic and heteroaromatic arylmetal substrates using N–H oxaziridine 18.
The aromatic and heteroaromatic metal reagents (25 and 26) were prepared using one of the following methods:
from aryl halides using activated Mg metal;
from aryl halides using the i-PrMgCl·LiCl complex (Knochel’s procedure);
performed with the aryllithium reagent via Li/halogen exchange;
the primary amination was performed with N–H oxaziridine 30;
direct C–H deprotonation with TMPMgCl·LiCl;
Li/Br exchange followed by transmetallation with MgBr2.