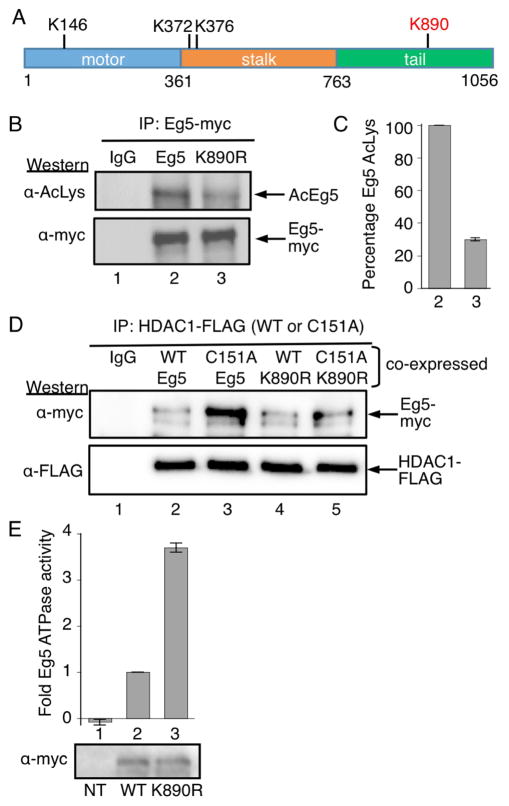
Figure 5.
Eg5 is deacetylated by HDAC1 at K890. A) The domain structure of human Eg5/KIF11, including the three acetylated lysines observed in prior work (K146, K372, and K376), as well as acetylated K890 identified here (red). B) Myc-tagged wild type or K890R mutant Eg5 were transfected into HEK293 cells, treated with HDAC1/2 selective inhibitor (SHI-1:2) for 24h, and immunoprecipitated with a myc antibody. SDS-PAGE separation and immunoblotting was performed with acetyl lysine (top) and myc (bottom) antibodies. C) Quantification of the Ac-Lys western blot from part B. Mean and standard error of four independent trials are shown (Figures S12A–E). Quantification of the myc blot as a load control is shown in Figures S11F–G. D) FLAG-tagged wild type (WT) or C151A mutant HDAC1 were cotransfected with myc-tagged wild type or K890R mutant Eg5 into HEK293 cells. Wild type and C151A mutant HDAC1 were immunoprecipitated using anti-FLAG agarose beads, separated by SDS-PAGE, and immunoblotted with myc (top) and FLAG (bottom) antibodies. E) Myc-tagged wild type or K890R mutant Eg5 were transfected into HEK293 cells, treated with HDAC1/2 selective inhibitor (SHI-1:2) for 24 hr to induce robust acetylation, and immunoprecipitated with a myc antibody. Half of the immunoprecipitate was used for ATPase assay (histogram) and other half was separated by SDS-PAGE and immunoblotted with myc antibody (gel image). Mean fold change and standard error are shown from at least three independent trials (Table S3). NT – non transfected. ***p<0.0001.