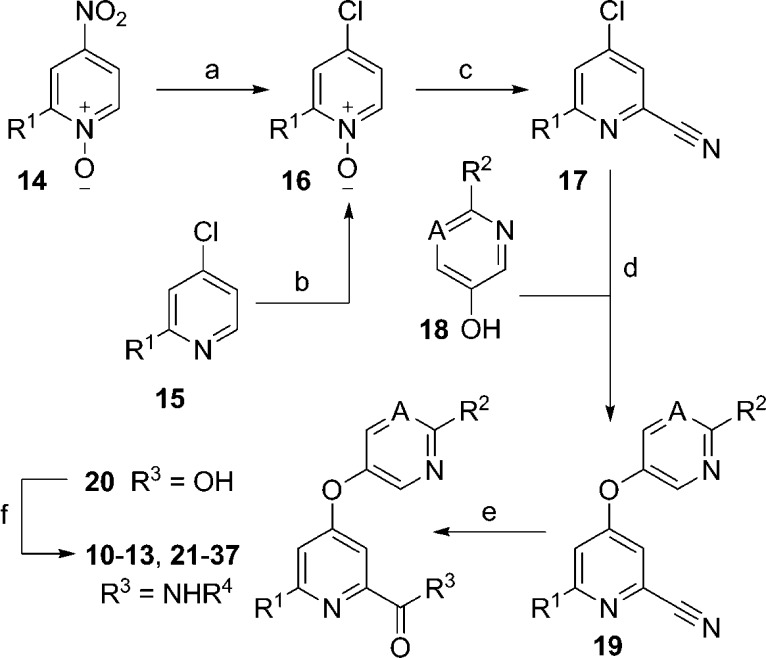
Scheme 1. Synthesis of Picolinamide Analogs 10–13 and 21–37.
Reagents and conditions: R1 = H, Me, CHF2; R2 = H, Me, C3H5; A = N, CH, CF, CCl; R4 = 4-methylthiazol-2-yl or substituted pyridin-2-yl; (a) conc. HCl, reflux, 53–75%; (b) H2O2·urea, (F3CCO)2O, THF, 0 °C to rt, 44–99%; (c) Me3SiCN, CH2Cl2, Me2NCOCl, 75–86%; (d) 18, K2CO3, DMF, 80 °C, 45–77%; (e) 2 N aq. NaOH, dioxane, reflux, 82–99%; (f) H2NR4, POCl3, pyridine, −15 °C, 22–81%.