Figure 5.
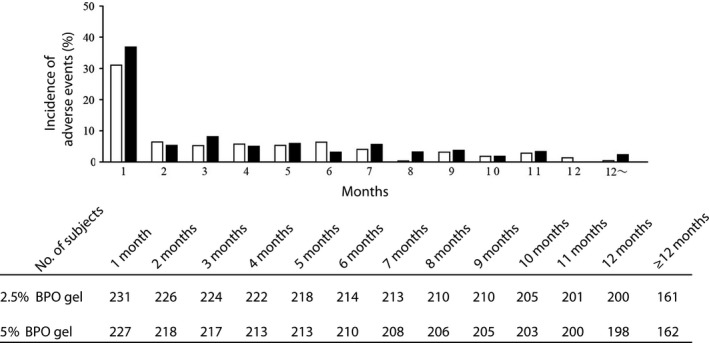
Reduction in the incidence of adverse reaction with a possible causal relation with the study drug over time. The incidence of new adverse events including repetitions that occurred in each period (1 month, 30 days) was graphed, and was represented as white (2.5% benzoyl peroxide [BPO] gel) and black (5% BPO gel) bars. Adverse events included here were irritation, erythema, pruritus and dryness at the application sites, skin exfoliation and contact dermatitis.
