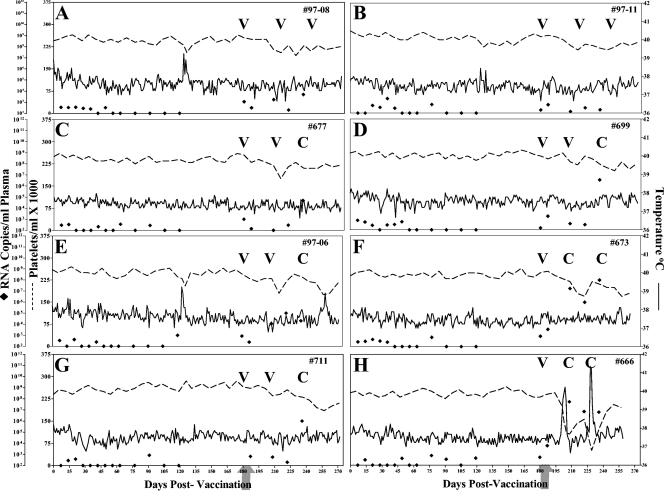
FIG. 2.
Clinical and virologic profiles of EIAVUKΔS2/DU experimental horses. Eight horses (A to H, respectively) were inoculated with EIAVUKΔS2/DU as described in Materials and Methods. Rectal temperature (solid line, right y axis) and platelet counts (dotted line, first left y axis) were monitored daily for up to 300 days (x axis) after the first vaccine dose. Quantification of the virus load (♦, second left y axis) was performed on viral RNA extracted from plasma at periodic time points prior to and after virulent virus challenge by using the LDME protocol (arrows). Febrile episodes (see panel A) were defined by a rectal temperature of >39°C in conjunction with a reduction in platelets (< 100,000/μl of whole blood) and other clinical symptoms of EIA. S2 diagnostic results for each animal are indicated in each respective panel above the date of analysis by either a V (vaccine strain) or a C (challenge strain).